Israeli regenerative medicine firm Matricelf has successfully tested a “first-of-its-kind” 3D printed spinal cord tissue implant on paralyzed mice that has enabled them to walk again.
Leveraging 3D bioprinting technology developed at Tel Aviv University (TAU), the firm successfully 3D printed and implanted a human tissue spinal cord implant into mice with both acute and chronic paralysis. All of the mice with acute paralysis regained their ability to walk, while the success rate of those with chronic paralysis was 80 percent.
With Matricelf now gearing up to enter into human trials with its 3D printed spinal cord implants by the end of 2024, the firm says a treatment for paralysis could potentially be only a few years away.
“This is a remarkable scientific achievement that represents the potential of Matricelf’s innovative and unique technology to develop complete autologous neural implants for patients with spinal cord injury,” said Asaf Toker, Matricelf’s CEO.
“The results of this study pave the way to future animal studies towards human clinical trials.”
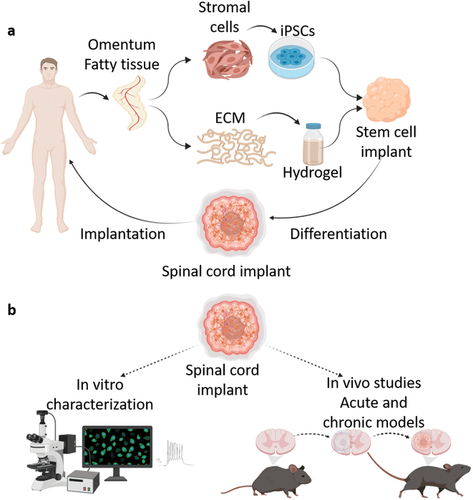
Matricelf’s 3D bioprinting technology
Matricelf’s latest development comes hot on the heels of signing an exclusive global licensing agreement with TAU’s technology transfer firm Ramot last month for a patent-pending 3D bioprinting technology. The technology has been in development for the last decade at Professor Tal Dvir’s Department of Biotechnology at TAU, who is also one of the founders of Matricelf and the firm’s Chief Scientific Officer.
The bioprinting technique works by simultaneously 3D printing cells and extracellular matrix (ECM) derived from patients to produce living human tissues and organs. The process leverages liquid nano-particles to stabilize the printed structures and ensure high resolution and precision, which are then extracted from the structure after printing.
The technique, which is currently undergoing the patent approval process in the US and Europe, was used to produce the “world’s first” 3D printed heart in 2019, complete with cells, blood vessels, ventricles, and chambers.
A potential cure for paralysis
Matricelf has published the details of its groundbreaking study in a new paper, which Dvir claims marks the first time in history that engineered human tissues have generated recovery in an animal model for long-term chronic paralysis.
With this currently being the most relevant model for paralysis treatment for humans, the study holds great promise for the development of future cures leveraging 3D bioprinting technologies.
The scientists began by taking a small biopsy of belly fat tissue from a patient containing cells and ECM. After separating the cells from the ECM, the team used genetic engineering to reprogram the cells and revert them to a state resembling embryonic stem cells capable of becoming any type of cell in the body.
One of the main issues with donor implants is their tendency to trigger an immune response and rejection after implantation. The team hoped to overcome this by using the patient’s ECM to form the basis of a personalized hydrogel within which the stem cells were encapsulated. The cells were then turned into 3D printed implants of neuronal networks containing motor neurons through a process mimicking the embryonic development of the spinal cord.
The spinal cord implants were then implanted in a group of mice, half of which had been recently paralyzed (acute paralyzation) and half of which had been paralyzed for the equivalent of a year in human terms (chronic paralyzation).
Following implantation and a rapid rehabilitation process, all of the mice with acute paralysis regained their ability to walk, compared to 80 percent of those with chronic paralysis.
Matricelf has entered into discussions with the US Food and Drug Administration (FDA), and is now planning the first human clinical trial of its 3D printed spinal cord implants at the end of 2024. In the meantime, the firm will continue to conduct additional efficacy and safety trials on lab rats.
“We hope to start clinical trials in humans within the next few years, and ultimately get these patients back on their feet,” said Dvir. “The innovative technology can serve as a platform for the generation of various engineered tissues for the treatment of additional medical conditions.”
Further information on the study can be found in the paper titled: “Regenerating the injured spinal cord at the chronic phase by engineered iPSCs-derived 3D neuronal networks,” published in the Advanced Science journal. The study is co-authored by L. Wertheim, R. Edri, Y. Goldshmit, T. Kagan, N. Noor, A. Ruban, A. Shapira, I. Gat-Viks, Y. Assaf, and T. Dvir.
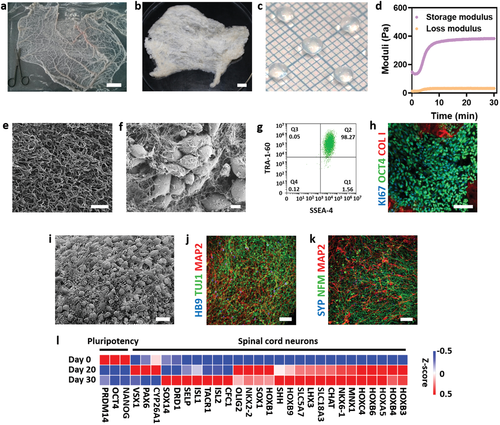
Advances in spinal cord treatments
As in many other regenerative medicine applications, 3D printing holds significant promise for improving and enhancing the success rates of spinal surgeries and treatments. While Matricelf’s breakthrough seems to be the furthest ahead in terms of realizing real-world human applications so far, there have been several other notable advances in this area in recent years.
Back in 2018, researchers at the University of Minnesota developed a prototype for a 3D printed scaffold with living cells that has the potential to help restore some function to patients with spinal cord injuries. Made from silicone and stem cells, the bioprinted scaffold could be placed within a patient’s spinal cord to serve as a kind of bridge between living nerve cells located around an injury. 2019, meanwhile, saw scientists from the University of California San Diego successfully 3D print a two milimeter spinal cord implant to repair spinal cord injuries in rats.
Regarding surgical applications, orthopedic implant firm 4WEB Medical added a stand-alone Anterior Spin Truss System to its 3D printed spin implant portfolio in 2020, designed to provide surgeons with greater confidence during spinal procedures.
Most recently, Texas-based 3D printed orthopedic device developer Orthofix Medical launched its new FORZA Ti PLIF Spacer System designed for use in Posterior Lumbar Interbody Fusion surgeries.
Subscribe to the 3D Printing Industry newsletter for the latest news in additive manufacturing. You can also stay connected by following us on Twitter and liking us on Facebook.
Looking for a career in additive manufacturing? Visit 3D Printing Jobs for a selection of roles in the industry.
Subscribe to our YouTube channel for the latest 3D printing video shorts, reviews and webinar replays.
Featured image shows the stages of Matricelf’s 3D bioprinted spinal cord process. Image via Matricelf.



