Californian medical device company SI-BONE, creators of the iFuse implant system, has announced FDA clearance and full U.S commercial launch of its 3D printed titanium implant, the iFuse-3D Implant.
The innovative medical implant was made using a metal 3D printer.
According to SI-BONE, the device is “the first-ever 3D-printed titanium implant for use in the SI joint.” The implant is designed to help patients who suffer from SI joint dysfunction.
The porous structure of the 3D printed implant is expected to improve bone growth, regrowth and through growth.
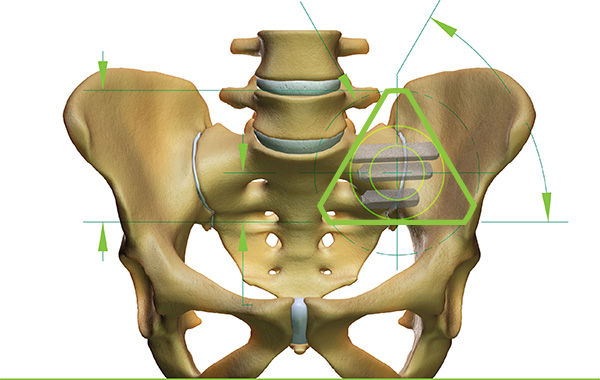
What is the SI joint?
The sacroiliac joint (SI joint) is located between the sacrum and the ilium bones in the pelvis. While the sacrum (in the center) supports the spine, the ilium bone supports the sacrum. It is for this reason that those who suffer from lower back pain often have SI joint dysfunction. According to SI-BONE, SI joint dysfunction is the cause of 15-30% of patients’ lower back pain.
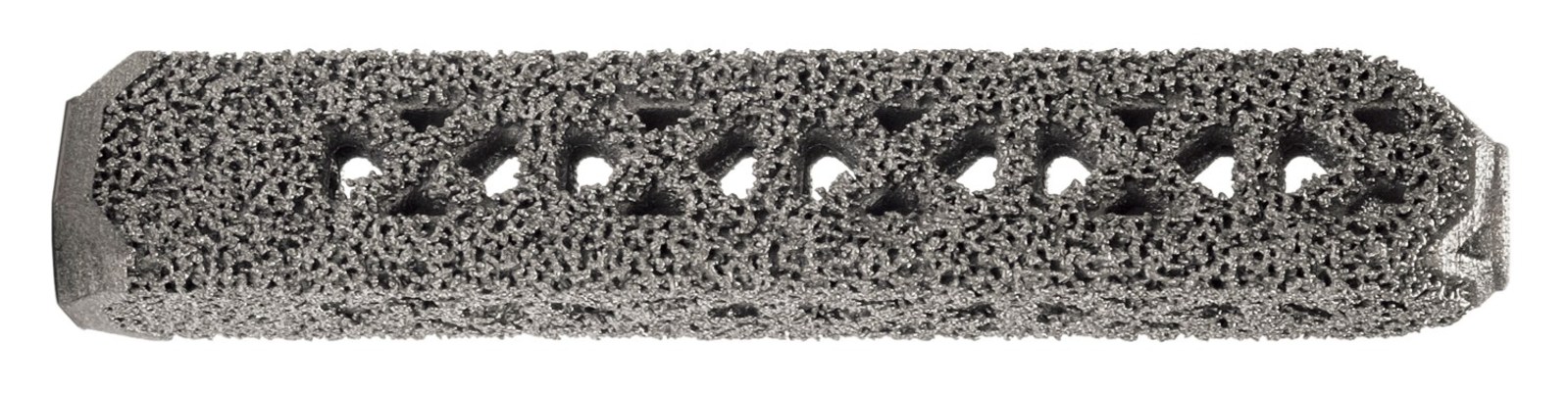
iFuse-3D implant
The iFuse 3D implant is based upon the clinically proven, patented, triangular design of the iFuse implant developed by SI-BONE. The non-3D printed iFuse implant has been used in over 26,000 procedures since 2009 and supported by more than 50 peer-reviewed publications.
A study published in the International Journal of Spine Surgery about the iFuse-3D shows how the implant can spur significant bone growth, regrowth and through growth.
The study reviews the implant of additive manufactured titanium devices into a flock of 24 sheep. The results are with more conventional TPS-coated devices. In conclusion, “the AM implants experienced significantly more bony infiltration compared to the TPS implants.”
As the study explains, the iFuse-3D implant was 3D printed using an Arcam EBAM machine. Arcam EBM was acquired by GE last and is the creator of a proprietary metal 3D printing method known as EBAM.
In an interview with 3D Printing Industry, the CEO of Arcam Magnus René gave further details of how Arcam works on medical implant projects like the iFuse-3D implant.
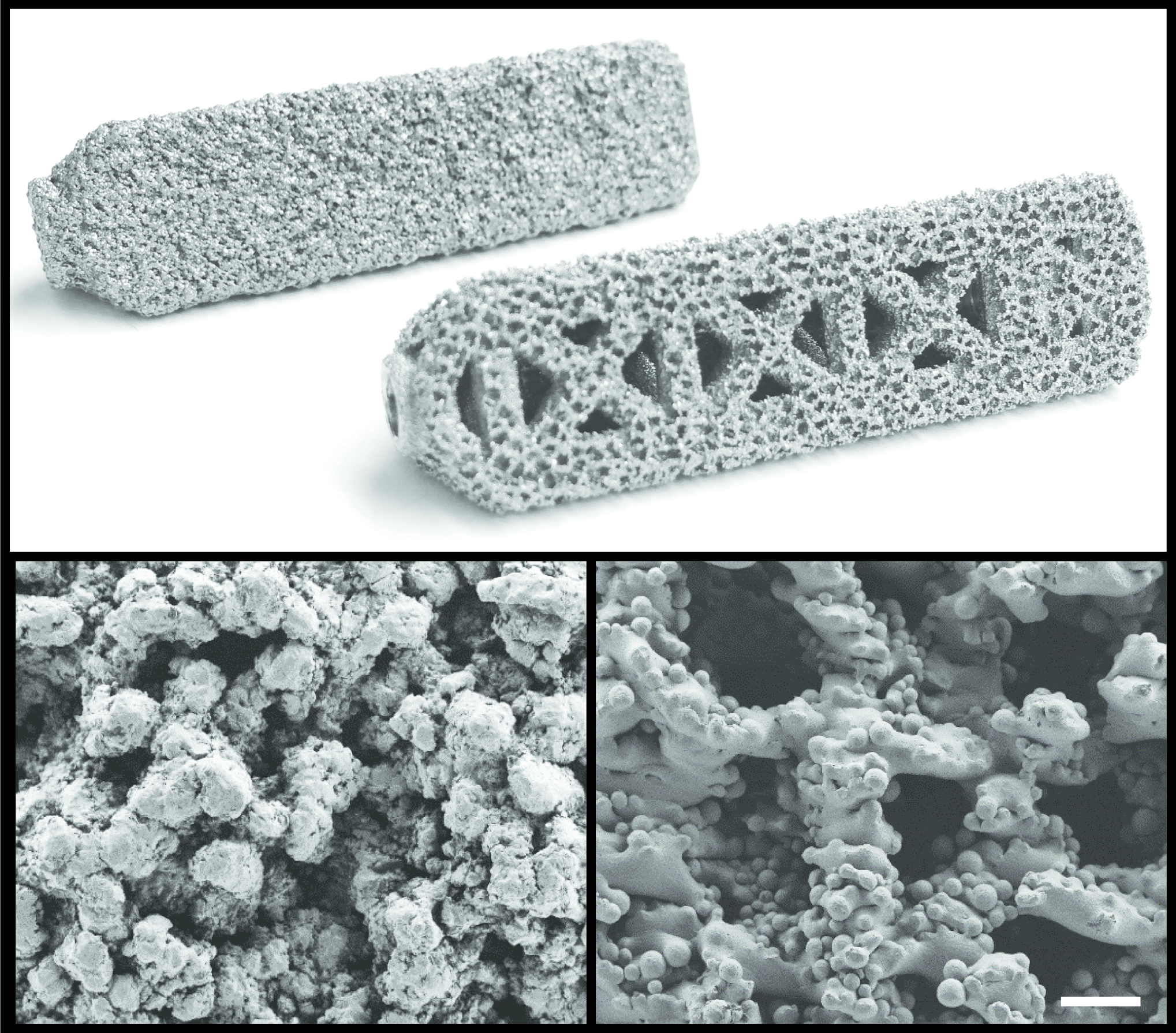
The benefits of 3D printed medical implants
Scott Yerby, CTO at SI-BONE, explains why the company turned to 3D printing,
Our goal was to expand the iFuse family using 3D-printing technology to provide enhanced surface characteristics while retaining key performance features of the iFuse Implant, including superior rotational resistance, mechanical strength and ease of use with our existing instrumentation. iFuse-3D, with its trabecular-like surface, provides 250% greater surface area than our highly successful iFuse Implant. Additionally, the structural fenestrations allow complete bone through growth.
The surface of these additive manufactured devices promotes bone regrowth. Titanium implants are also advantageous as seen in the case of an Australian woman receiving a jaw implant.
Elsewhere in the medical world, surgeons in China used 3D printing to create a porous breast implant for a cancer patient.
For the latest new about how 3D printers can be used in medicine, subscribe to the most widely read newsletter in the 3D printing industry, follow us on twitter and like us on Facebook.
Featured image shows the triangular design of the iFuse 3D implant displaying bone growth. Image via PRNewsfoto/SI-BONE, Inc.


