Biomaterials platform company Dimension Inx has announced that it has closed a $12M Series A Round.
Prime Movers Lab (PML) led the round, with cooperation from returning investors KdT Ventures and Revolution’s Rise of the Rest Seed Fund (ROTR). Solas BioVentures, Portal Innovation Ventures, and Alumni Ventures are among the new investors in this round. The funds will be employed to accelerate the development of functional organ regeneration therapeutics and to broaden the company’s manufacturing capabilities. The funds will be used to assist in the commercial release of the firm’s first product, CMFlex, in the near future.
Dimension Inx also revealed that CMFlex, the first 3D printed restorative bone graft product approved by the FDA, has obtained 510(k) clearance from the US Food and Drug Administration (FDA). CMFlex, a ready-to-use versatile ceramic for oral and maxillo-facial indications, enhances clinician workflow and decreases surgical case time whilst also restoring healthy bone.
“Dimension Inx represents the type of breakthrough science we believe will transform their industry and reshape our physical world,” said Prime Movers Lab Venture Partner Amy Kruse, Ph.D. who will be joining the company’s board. “We’re excited to support Dimension Inx as they enter this next phase of growth and development, and bring their transformative products to market.”
“FDA clearance is a major milestone for our platform, and validates the work we’ve put into developing our product over the past two years,” said CEO of Dimension Inx, Caralynn Nowinski Collens, M.D. “CMFlex is a product that represents our unique approach to restoring functionality in the body: it’s a dynamic collaboration between biology, material composition, microstructure, and macro architecture. We’re excited by the interest we’ve already received from surgeons who recognize the importance of a ready-to-use solution that is easy to handle and accelerates healing to improve patient outcomes.”
Dimension Inx’s industrial 3D printing portfolio
Previously, a team of Carnegie Mellon University (CMU) and University of Connecticut (UConn) researchers 3D printed novel calcium phosphate graphene (CaPG) scaffolds that could be utilized for bone restoration applications in the future. Dimension Inx’s Direct Ink Writing (DIW) 3D printing method was used by the team to print porous concepts of its CaPG material with a large weight fraction to allow a “significantly high” graphenic content inside the ink (around 90%). This allowed cellular access to the osteoconductive backbone and the sustained release of phosphate and calcium ions, indicating that the operational graphenic content, instead of the bioinert binder, dominated the host’s response to the matrix.
“We pride ourselves in catalyzing a step function change to unlock the full potential of regenerative therapeutics to cure complex disease, and we’re grateful to do so with the support of our investors,” added Dr. Collens. “Our approach has always focused on the entire tissue microenvironment. If we provide the right blueprint, we make it possible to recruit the body’s natural strengths and accelerate the repair, regeneration, and ultimately, restoration of critical body function.”
Furthermore, the US Department of Defense (DoD) granted Dimension Inx $240,000 in funding to build a regenerative medical system capable of restoring tracheal injuries. The project used the company’s patented biomaterials platform to produce implantable parts that mimic the tracheal microenvironment and merge them with a novel hydrogel to assist in remodeling into typically functional tracheal tissue.
Series funding rounds that were carried out in the additive manufacturing sector
Previously, San Francisco-based on-demand manufacturing service provider Fictiv raised $100 million in Series E funding. This round of funding increased the firm’s total capital raised to about $192 million. Activate Capital led Fictiv’s Series E round, with participation from established institutional investors Accel, Bill Gates, G2 Venture Partners, and Standard Investments. Angeleno Group, Cross Creek, and Westly Group joined as new investors.
Elsewhere, Swiss carbon fiber 3D printing specialist 9T Labs raised $17 million in Series A funding, which it used to commercialize its Red Series Additive Fusion Solution platform. The platform, which combines 3D printing and compression molding, is intended for the creation of advanced carbon fiber-reinforced thermoplastic composite parts in quantities varying from 100 to over 100,000 parts per year. With the support of industrial 3D printer manufacturer Stratasys, as well as other key investors, 9T Labs sought to fully commercialize the platform to produce components for end-use applications in the aerospace, automotive, medical, industrial, and consumer goods industries.
What does the future of 3D printing for the next ten years hold?
What engineering challenges will need to be tackled in the additive manufacturing sector in the coming decade?
To stay up to date with the latest 3D printing news, don’t forget to subscribe to the 3D Printing Industry newsletter or follow us on Twitter, or like our page on Facebook.
While you’re here, why not subscribe to our Youtube channel? Featuring discussion, debriefs, video shorts, and webinar replays.
Are you looking for a job in the additive manufacturing industry? Visit 3D Printing Jobs for a selection of roles in the industry.
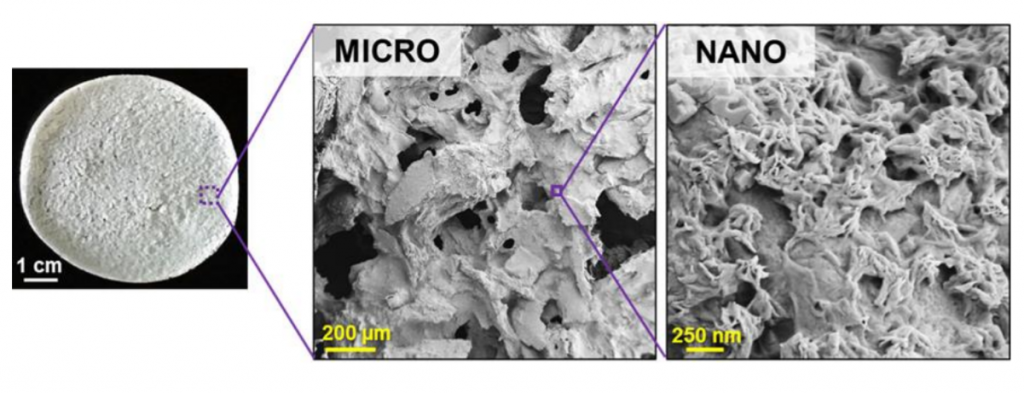
Feature image shows a representative example of a small sheet of Tissue PaperTM derived from ovarian tissue, highlighting its unique micro- and nano-porosity and texture. Image via Dimension Inx.



