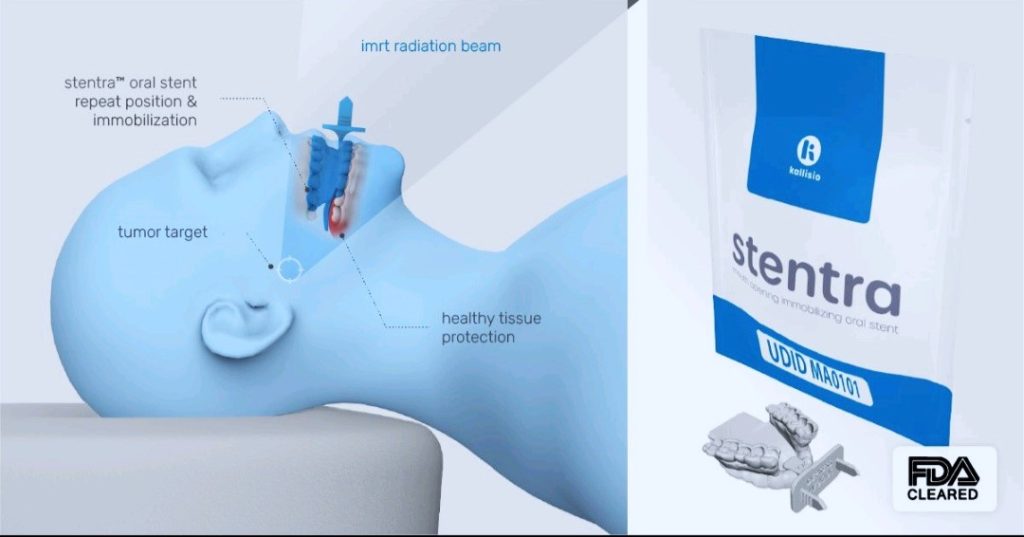
Medical equipment manufacturer Kallisio has announced the FDA 510(k) clearance for its Stentra, a 3D printed oral stent tailored for Head and Neck Cancer (HNC) patients undergoing radiation therapy.
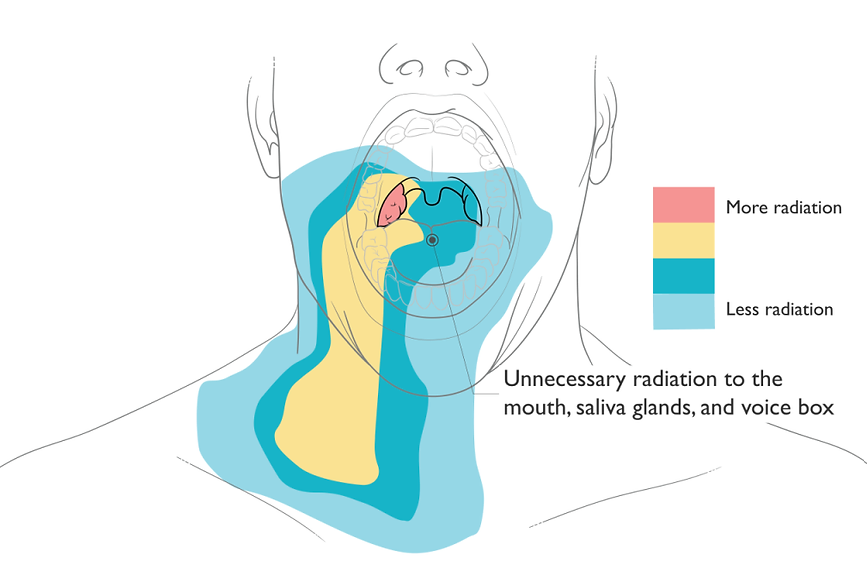
Utilizing personalized 3D printing technology, the Stentra device is crafted to address toxicity concerns, safeguarding healthy tissue around the target tumor area. The customization process integrates each patient’s oral data with 3D printing, aiming to reduce common target positioning discrepancies found in radiotherapy methods like Intensity-Modulated Radiation Therapy (IMRT). Specifically designed to immobilize critical organs at risk (OARs), such as the tongue and lips, the oral immobilization stent prevents exposure to the high-dose radiation target zone.
“Designed to address each patient’s unique treatment and anatomical needs, Stentra delivers an unmatched level of customization and effectiveness in addressing the critical need to safeguard healthy tissue during radiation therapy,” says Rajan Patel, Kallisio Co-founder and CEO. “FDA clearance is an exciting milestone in our journey to improve patient experiences and quality of life.”
A transformative approach to cancer treatment
One significant advantage of Stentra is its emphasis on high treatment accuracy, providing a patient-specific solution for precise and targeted radiation therapy. Focusing on effective toxicity management, this individualized treatment aims to enhance efficacy while minimizing potential side effects by preserving healthy tissue from the adverse impact of radiation. The expedited fabrication process of patient-tailored oral stents in less than five days contributes to the efficiency of the technology.
The underlying technology of Stentra was licensed by the University of Texas MD Anderson Cancer Center and developed by Dr. Eugene Koay, an Associate Professor of Gastrointestinal Radiation Oncology. Dr. Koay is also involved with Kallisio in an advisory capacity. It is important to note that a conflict of interest exists between Kallisio and MD Anderson, which is being managed according to an MD Anderson Conflict of Interest Management and Monitoring Plan.
Kallisio is actively collaborating with cancer centers in the United States to introduce Stentra. The collaboration involves intraoral scanning equipment and a clinical portal ordering system, ensuring real-time monitoring, compliance, and integration into the radiation oncology workflow.
In the past, Pohang University of Science and Technology (POSTECH) researchers developed 3D printed stents using a throat cell-derived bio-ink, easing esophagitis symptoms in radiotherapy patients. Incorporating a ‘rotating rod’ system, the dumbbell-like devices were able to reduce inflammation caused by cancer therapies.
Loaded with hydrogel and tested successfully in an immortalized human model and esophagitis-induced lab rats, the stents demonstrated over 90% cell viability. According to the team, these stents could treat various inflammation-based injuries, offering a promising therapeutic solution.
Streamlining cancer treatment with 3D printing
Cancer treatment has come a long way by leveraging 3D printing technologies. A recent example includes an alliance between CELLINK and Carcinotech, aimed at fast-tracking cancer drug development by developing advanced protocols for the biofabrication of 3D bioprinted tumor models using cancer cell lines.
Created to enhance accuracy, cut development costs, and boost research output, these models offer researchers efficient tools for drug development and an improved comprehension of cancer biology. The goal is to streamline and expedite the drug development process using these 3D bioprinted tumor models.
Claudius Regaud Institute and Toulouse University Hospital achieved a facial transplant by growing a 3D bioprinted nose on the patient’s forearm. Bone graft 3D printing specialist CERHUM contributed with its MyBone technology, utilizing fully biocompatible bioceramic for successful facial bone grafting via 3D printing.
Developed for a patient who lost her nose due to cancer treatments, the unique procedure involved crafting a biomaterial-based, nose-shaped structure, which was implanted on the forearm for two months. The implant was revascularized through microsurgery and successfully transplanted onto the patient’s face.
What does the future of 3D printing for the next ten years hold?
What engineering challenges will need to be tackled in the additive manufacturing sector in the coming decade?
To stay up to date with the latest 3D printing news, don’t forget to subscribe to the 3D Printing Industry newsletter or follow us on Twitter, or like our page on Facebook.
While you’re here, why not subscribe to our Youtube channel? Featuring discussion, debriefs, video shorts, and webinar replays.
Are you looking for a job in the additive manufacturing industry? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured image shows Kallisio’s FDA-cleared Stentra. Photo via Kallisio.



