Ahead of Formnext 2023, 3D Printing Industry spoke to the winners of the Formnext Start-Up challenge.
In a recent conversation, Ivana Llobet, CEO and co-founder of Odapt, delved into her professional trajectory and the evolution of her latest project. Hailing from Barcelona, Llobet began her biomedical engineering academic journey, later specializing in healthcare design in London. She said, “I specialized more in the design aspect within healthcare, how to identify problems, how to talk to users, co-create with them.” Post-London, her pursuit of knowledge led her to Boston, where she ventured into the pharma sector before focusing on product design in Barcelona.
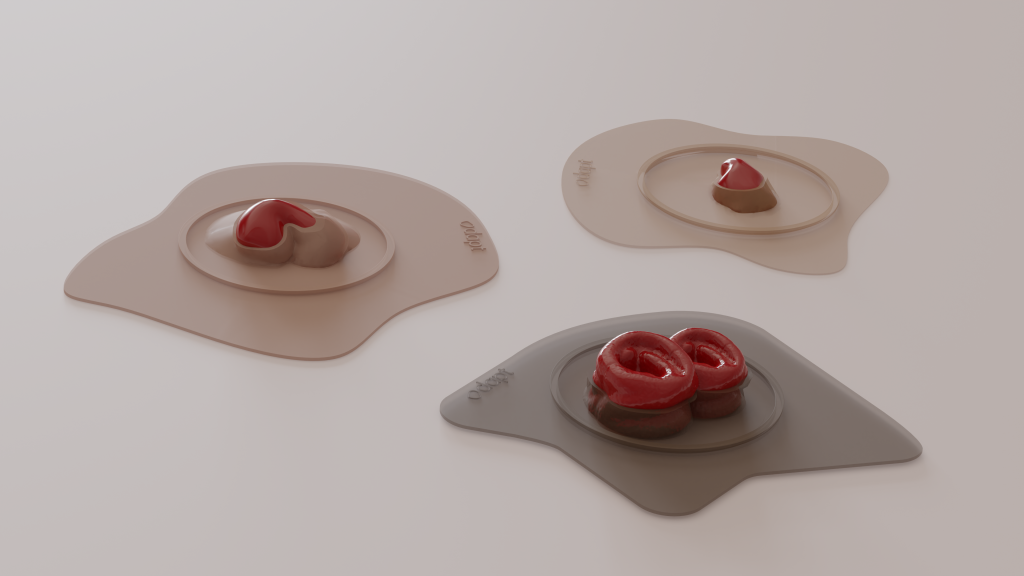
Odapt’s mission addresses the daily challenges faced by ostomy bag users, particularly leakage. Llobet elaborated, “The main problem with these [ostomy bags] is leakage. We’re trying to eliminate leaks by doing personalized wafers.” Designed to match an individual’s anatomy perfectly, these wafers offer an effective solution to the one-size-fits-all approach of current ostomy bags.
An ostomy bag, also known as a stoma bag or pouch, is a medical device used to collect waste from a surgically created opening (stoma) in the body. This opening can result from various surgical procedures that divert bodily waste from its usual path due to medical conditions or diseases that have damaged or affected normal function. There are different types of ostomies, each relating to the part of the body involved.
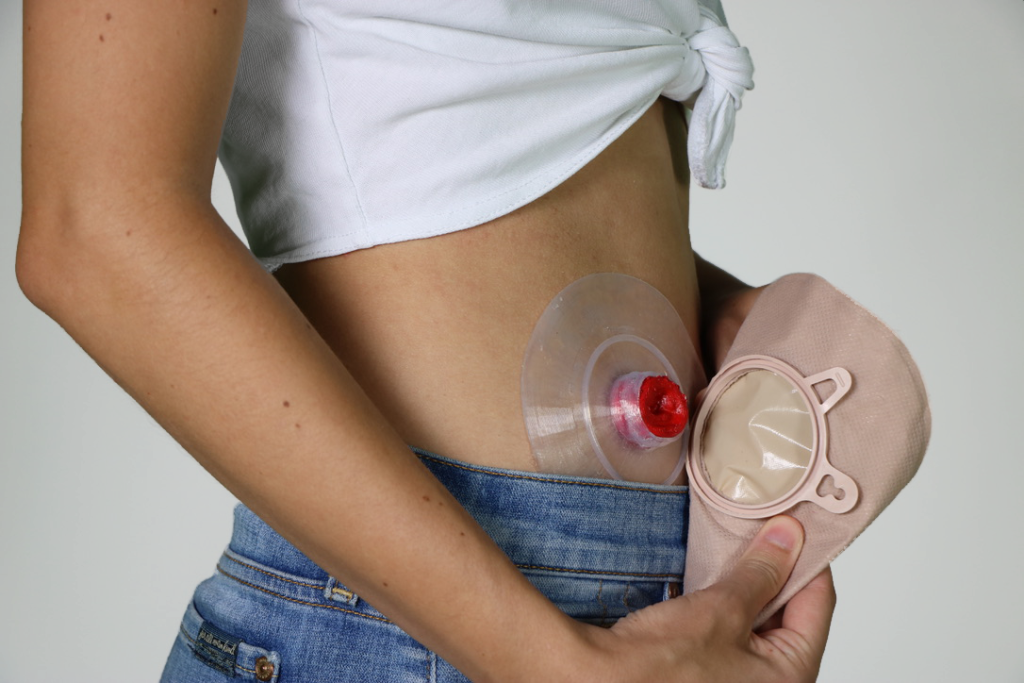
On her initial encounter with an ostomy bag user in London, Llobet recalls her surprise: “I come from a healthcare background and the med tech industry, and I have never heard about ostomy bags.” This encounter made her question the product’s stagnant design since its inception in the 1950s. Regarding the current market, Llobet noted the outdated process: “People need to cut the wafer every day manually… it’s a very complex [process], and it just doesn’t work well.” Odapt’s primary focus is on younger users, aiming to improve their experience before moving on to addressing the needs of elderly users.
More than 13 million people globally rely on ostomy bags, with Spain alone accounting for one in 450 individuals using the medical device, stated Llobet. Despite the prevalence, she commented on the lack of awareness, “There’s very little awareness on this condition, very little awareness on ostomy bags just because it’s invisible and it’s taboo.” In a groundbreaking move, Odapt is the first to apply 3D printing for these devices. Llobet enthusiastically revealed, “We’re really applying 3D printing…and we’re excited about it.” The company offers a personalized solution, allowing customers to scan their stomas using a mobile app and modify the design according to their unique requirements. They are using 3D printing with biocompatible silicone via a method known as liquid deposition modeling, currently working with Elkem Silicones and Lynxter.
The CEO and co-founder highlighted the severe health consequences of traditional wafers used in ostomy bags, noting, “It’s not solid stools, it’s liquid. It’s gastric acid.” She elaborated on the distressing effects of such leaks, stating that they cause “burning and irritating” of the skin, leading some patients back to hospitals or even necessitating additional surgeries. These leaks represent physical discomfort and a “constant anxiety” for users, especially in public settings. Ahead of their appearance at Formnext, Llobet emphasized the company’s drive to collaborate with the 3D printing industry to source “certified biocompatible materials, certified machines, and certified companies.” This effort aims to explore new advancements in silicon and raise awareness about their project and the broader challenges of ostomies.
The shortlists are online for the 3D Printing Industry Awards 2023. Cast your vote now!
Addressing the deficiencies in ostomy bags
The customized 3D printed ostomy bags from Odapt promise a superior fit and a sustainable solution to a prevalent medical need. Llobet highlighted the significance of personalization, saying, “People normally change their bags every day.” With their new design, the product’s silicone composition allows easy cleaning and sterilization by boiling, much like a menstrual cup. This adaptability offers an impressive feature, as Llobet explained: “It has like this memory shape that it’s going to stay exactly where it was.” However, venturing into this sphere is challenging. She acknowledged the regulatory landscape, stating, “It’s a medical device. And with this comes a lot of regulations.” While Europe categorizes it as a class two medical device requiring clinical trials, the US deems it a class one, alleviating the need for such trials.
Odapt is poised to initiate a clinical trial in Barcelona, indicating a strategic approach towards its commercial launch. Llobet elaborated on their plan, “Our idea is to start a clinical trial in 2024, in Barcelona.” Notably, she mentioned the inherent advantages of 3D printing, stating, “People could be anywhere in the world, and we could print it locally in their countries and send it to them.” As for technological explorations, Llobet disclosed, “We’ve also been considering creating molding systems.” One significant hurdle they’ve tackled is the pouch’s different hardnessess.
Leading a medical-focused start-up
As an innovative startup developing 3D printed healthcare solutions, Odapt has faced a slew of challenges in its development phase. Llobet highlighted that their primary concern was achieving the right balance between strength and flexibility of the product. “The main challenge was to have this feeling…it’s strong enough [yet] flexible,” she stated. She emphasized the adhesive component’s pivotal nature, noting, “The adhesive part was key.” They’ve found silicone to be the best fit for the application.
On the corporate front, Odapt comprises three co-founders – Ivana Llobet, Jessica Nissen, and Patricia López – and recently welcomed another member, Mario García. The team plans to operate simultaneously from the USA, California, and Barcelona in Spain. Currently, their funding model relies significantly on competitions, as Ivana remarked, “We won the BASF competition on 3D printing, and we won 25,000 euros.” The company aims to secure additional financing for the impending clinical trial in Spain.
As they venture into the US market, understanding the regulatory ecosystem, especially the intricacies of the FDA, becomes crucial. Llobet highlights a multi-faceted challenge in medical 3D printing, “You need to certify not only the machine and the material but the whole process.” She emphasizes their focus on ostomies, with designs and new ideas for the wafer and the whole ostomy bag experience. On exploring other applications using 3D printed biocompatible silicone, Llobet mentions another project involving a “neonatal ventilation mask for neonates led by Mario García.” As a startup, navigating the regulatory burdens is daunting. Llobet suggests that feedback from regulatory organizations, similar to the FDA’s approach, could prove beneficial, especially for startups. She notes, “This feedback from the regulatory organization, would be very beneficial, especially for startups.”
Regarding the regulatory differences between Europe and the US, there’s a rising trend of medical device companies approaching the US FDA before navigating Europe’s regulatory system. She mentioned, “Many people are turning to the FDA first because of this feedback…But it’s happening now that in the US, maybe you’re class one, and in Europe, you’re class two.” As for the product’s introduction post-regulatory approval, Llobet envisages it will be covered by insurance. She stated, “In Europe people pay 10% of the whole price.” She remains optimistic about its insurance inclusion, underscoring potential benefits such as “reducing surgeries, reducing infections, or improving the quality of life.”
Make sure to visit Odapt during Formnext 2023, find them at Hall 11.1 Stand B55D.

Read more in our executive interview series with 3D printing leaders here.
What does the future of 3D printing for the next ten years hold?
What engineering challenges will need to be tackled in the additive manufacturing sector in the coming decade?
To stay up to date with the latest 3D printing news, don’t forget to subscribe to the 3D Printing Industry newsletter or follow us on Twitter, or like our page on Facebook.
While you’re here, why not subscribe to our Youtube channel? Featuring discussion, debriefs, video shorts, and webinar replays.
Are you looking for a job in the additive manufacturing industry? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured image shows Odapt co-founders Jessica Nissen, Ivana Llobet and Patricia López. Photo via Odapt.



