The votes for our annual awards ceremony are now in! Watch the 2022 3D Printing Industry Awards replay to find out this year’s winners.
3D bioprinting specialist CollPlant has added a new bio-ink to its material portfolio.
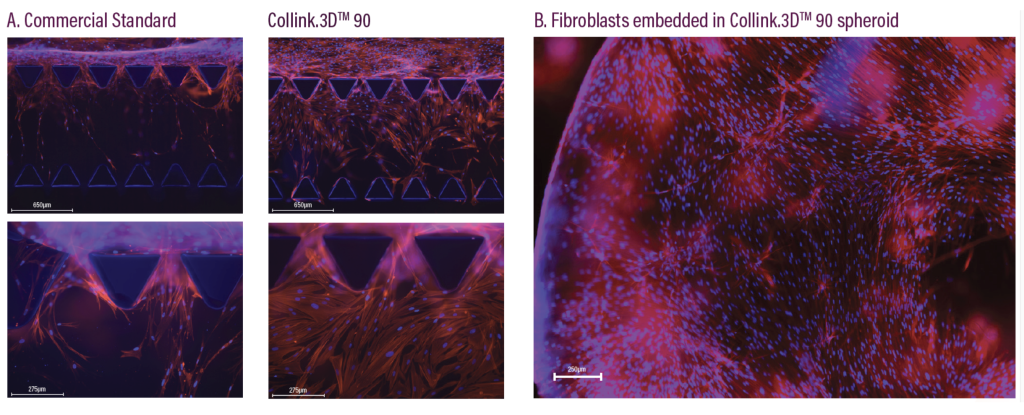
Known as ‘Collink.3D 90,’ CollPlant’s second recombinant human collagen (rhCollagen)-based material is said to feature enhanced mechanical properties that directly address the print requirements of hard and soft tissues. Compared to existing cell-culturing hydrogels, the ink is also said to facilitate faster cell migration, a benefit that could make it an ideal basis for developing regenerative medicines.
“We are very excited to continue to broaden our rhCollagen-based bio-ink offerings, providing our biopharma and academic customers the ability to print a variety of applications,” said CollPlant CEO, Yehiel Tal. “In addition to our product pipeline, bio-inks are an important and strategic platform that supports CollPlant’s efforts to pursue licensing and collaboration agreements with business partners, and we will continue to introduce new bio-inks and innovate in this area.”
“We believe our bio-inks deliver a favorable alternative to existing commercial bio-inks owing to their high bio-functionality, rheological properties and high purity.”
CollPlant’s collagen-growing technology
CollPlant’s offering revolves around a proprietary plant-based genetic engineering technology, which it markets as a means of developing regenerative medicines and medical aesthetic treatments. In essence, the firm’s approach sees it grow collagen on the leaves of genetically-engineered tobacco plants that are later harvested and processed into a highly-purified 3D bioprinting material.
Collagen, which is seen as a vital building block in the creation of regenerative medicines, is currently extracted from animal and human cadavers. However, CollPlant says this process is fraught with contamination and allergy risks, while subjecting natural collagen to harsh purification can lead to “irreversible modifications” that “impede its biofunctionality.”
By contrast, the company’s animal-free alternative is said to be identical to that generated inside the human body, while also boasting enhanced biofunctionality, high homogeneity, and offering up fewer risks in terms of adverse responses.
In the past, CollPlant has partnered with 3D Systems to deploy its rhCollagen bio-inks within tissue bioprinting applications. United Therapeutics’ deal to license CollPlant’s technology two years ago also saw it used to produce materials for the serial 3D printing of kidneys, while earlier this year, CollPlant itself revealed that the 3D bioprinted breast implants it has developed are entering animal trials.

Bringing Collink.3D 90 to market
Designed to build on the platform laid by CollPlant’s Collink.3D 50 bio-ink, Collink.3D 90 enables the faster migration of cells into gel matrices, in a way that maximizes cell viability in 3D bioprinting applications. According to the firm, cell migration is pivotal to tissue repair, thus compared to many commercial hydrogels, its new material could offer “a superior, animal free, human cell culture substrate.”
The bio-ink is based on a collagen which has been modified so that 90% of its primary amines, molecules used to carry out metabolic and physiological functions in the body, have been turned into polymeric methacrylamides. It’s this high level of functionalization that results in a stiffer gel, with improved mechanical properties.
CollPlant adds that given the ability of its newly-expanded Collink.3D range to yield high-resolution, scalable 3D bioprinted scaffolds that accurately mimic the properties of human tissues, they could also access other applications.
These include cell culturing, tissue modeling and drug discovery, as well as tissue engineering and even implantable tissues, an area the company sees as a potential multi-billion-dollar opportunity. More broadly, due to its wide compatibility with 3D bioprinters and various cell types, including stem cells, pluripotent stem cells, endothelial, and epithelial cells, CollPlant says its new bio-ink offers “superior biological performance, consistency and safety” to researchers across the industry.
Though implants, particularly organs, produced via 3D bioprinting still face numerous challenges before they can be deployed at any kind of scale, the technology continues to make advances at an experimental level. Earlier this year, 3DBio Therapeutics and the Microtia-Congenital Ear Deformity Institute completed a human ear reconstruction using the former’s 3D printed AuriNovo tissue implant.
More recently, clinicians at the Claudius Regaud Institute and Toulouse University Hospital have put the technology into practice, by treating a cancer patient with a 3D bioprinted nose grown on their arm. Having been implanted and fostered on the patient’s forearm for two months, the bioprinted structure was removed, revascularized and implanted onto their face, as a part of a breakthrough surgery.
To stay up to date with the latest 3D printing news, don’t forget to subscribe to the 3D Printing Industry newsletter or follow us on Twitter or liking our page on Facebook.
While you’re here, why not subscribe to our Youtube channel? featuring discussion, debriefs, video shorts and webinar replays.
Are you looking for a job in the additive manufacturing industry? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured image shows a graphic demonstration of 3D bioprinting with a rhCollagen bio-ink. Image via CollPlant.



