Researchers from the Massachusetts Institute of Technology (MIT) have used fine-scale 3D bioprinting and melt electrowriting to grow highly uniform cell cultures.
This method, presented in the journal Microsystems and Nanoengineering, was created to produce lattice scaffolds that enable precise control over its environment as well as cultured cells with particular characteristics.
“If you take cells and put them on a conventional 3D printed surface, it’s like a 2D surface to them, because the cells themselves are so much smaller,” explained Filippos Tourlomousis, a postdoctoral associate at MIT’s Center for Bits and Atoms, and first author of the research.
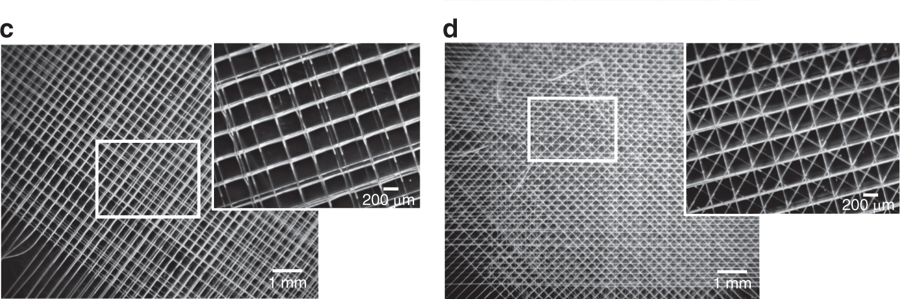
“But in a mesh-like structure printed using the electro-writing method, the structure is at the same size scale as the cells themselves, and so their sizes and shapes and the way they form adhesions to the material can be controlled by adjusting the porous microarchitecture of the printed lattice structure.”
3D bioprinting and melt electro writing
Tourlomousis and six others at MIT and the Stevens Institute of Technology in New Jersey sought out to accurately produce and tune cells in order to observe and control cell phenotypes; this is due to the need for tighter control over cellular function. According to the researchers, this is a major roadblock for getting tissue engineering products to the clinic.
“Any steps to tighten specifications on the scaffold, and thereby also tighten the variance in cell phenotype, are much needed by this industry,” added Tourlomousis.
“While ordinary 3D printing produces filaments as fine as 150 microns (millionths of a meter), it’s possible to get fibers down to widths of 10 microns by adding a strong electric field between the nozzle extruding the fiber and the stage on which the structure is being printed.” This method is known as melt electrowriting.
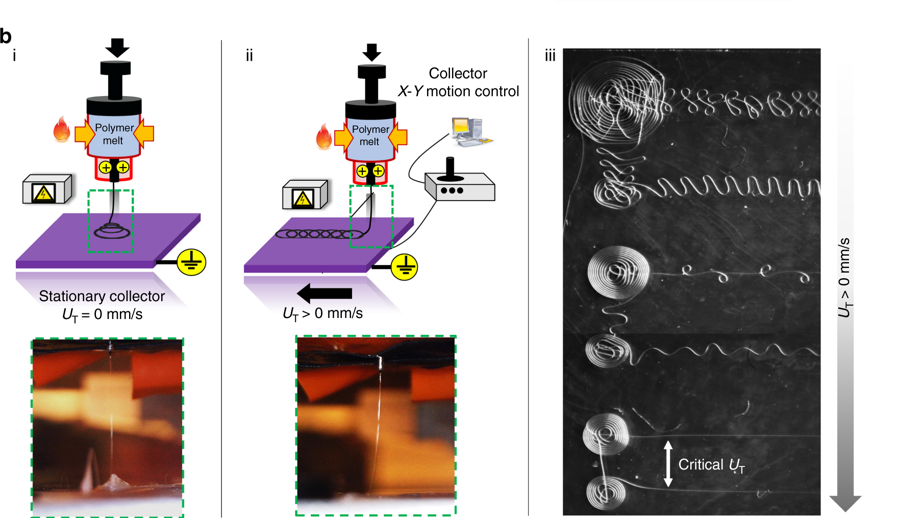
The study explains that cells form proteins known as focal adhesions at the places where they attach themselves to the structure. “Focal adhesions are the way the cell communicates with the external environment. These proteins have measurable features across the cell body allowing us to do metrology. We quantify these features and use them to model and classify quite precisely individual cell shapes.”
Tourlomousis stated, “It is widely known that cell shape governs cell function and this work suggests a shape-driven pathway for engineering and quantifying cell responses with great precision and with great reproducibility.”
The researchers believe this method could be used to 3D print metamaterials that can produce rare optical or electronic properties.
“Machine learning metrology of cell confinement in melt electrowritten three-dimensional biomaterial substrates” is co-authored by Thrasyvoulos Karydis, Andreas Mershin, Chao Jia, Hongjun Wang, Dilhan Kalyon, and Robert Chang.
Voting for the 2019 3D Printing Industry Awards is now open. To see if your nominations made the shortlist, and to help decide this year’s winners, cast your votes now.
For the latest additive manufacturing research, subscribe to the 3D Printing Industry newsletter, follow us on Twitter and like us on Facebook.
Looking for a career in additive manufacturing? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured image shows Microfilaments made using a new 3D printing method, shown in gray in this illustration, form a structure that cells, shown in color, can adhere to. The shapes formed by the filaments determine the very uniform shapes of cells. Image via Eli Gershenfeld/MIT.


