Korean 3D bioprinter manufacturer T&R Biofab has successfully fabricated liver tissues and transplanted them into an animal test subject for the first time.
Using one of the firm’s modified 3DX bioprinters, its researchers have been able to pattern spherical microtissues into structures, which replicate those of the ‘lobules’ found inside the human liver. Once implanted into lab mice, the resulting ‘micro-organs’ have shown excellent viability and structural stability, potentially making them a significant step towards the regenerative liver therapies of the future.
“What our research has highlighted for the first time is that 3D bioprinting can actually make a difference in [cellular] 3D structures,” said Paulo André Marinho, Head of Scientific Strategy at T&R Biofab. “We’ve made a phenotypically-relevant tissue that, once injected into animals, engrafts substantially better than 3D counterparts without structure.”
“We seem to be the first to successfully fully-bioprint highly-organized constructs that, once transplanted, had nearly no cell death or fibrotic tissue observed.”
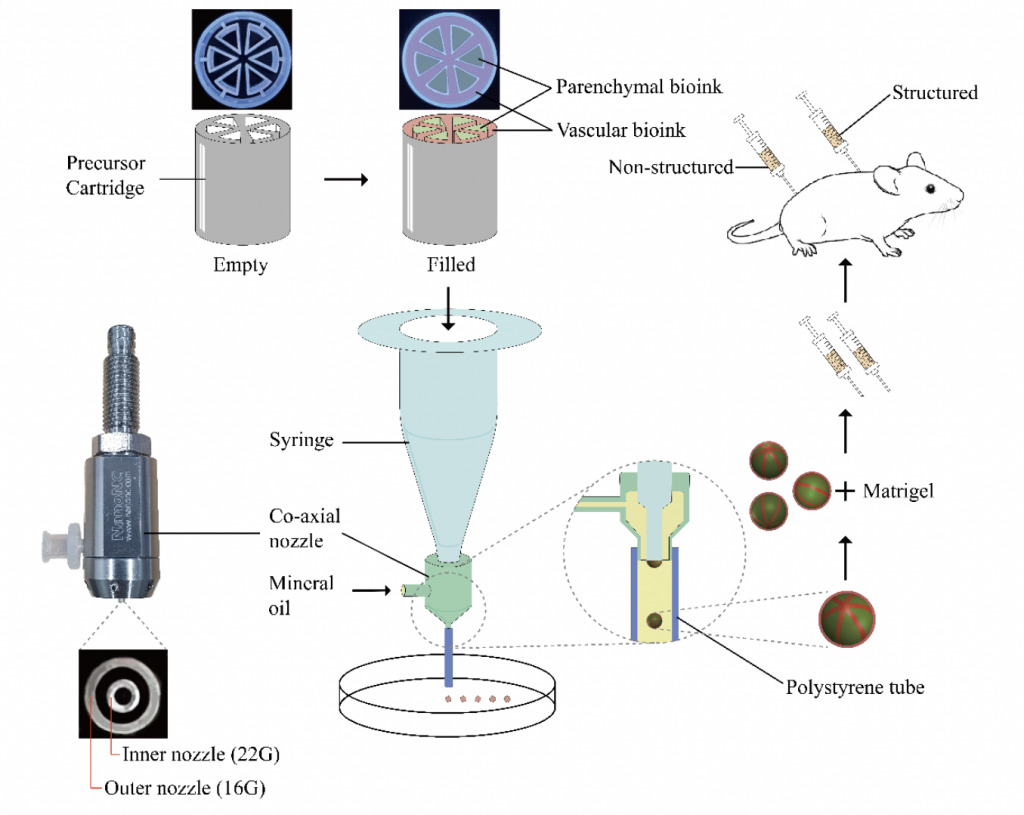
Bioprinting liver lobules
Generally speaking, the human body is composed of several different multiscaled tissues and organs, with the liver being a particularly highly-vascularized example. Of the human liver, around 80% is made up of small functional units called hepatic lobules, and advances in 3D bioprinting are increasingly making it possible to replicate these building blocks, and create thicker, more viable soft tissue models.
However, cultivating these hepatocytes continues to prove difficult, particularly when attempting to bioprint organs with sufficient vascularization and cell viability for potential transplantation. One of the main drawbacks to bioprinting lobules lies in the technology used to create them, as UV-based approaches often require the use of cross-linker which can be toxic to liver cells, damaging their viability.
Likewise, where livers have been bioprinted on a microscale and transplanted in previous studies, they’ve only kept animal test subjects alive for 5 to 25 extra days, limiting the human applications of such methods. To get around this, and develop a means of producing more viable tissues with a greater level of cell placement precision, T&R Biofab’s team developed a novel extrusion technique last year.
In effect, the researchers’ revised method involved using a lobule-shaped cartridge with slots for hepatocyte cells, endothelial cells and cell-free bio-inks to produce vascularized structures, but extruding these materials into 3D layers still proved troublesome, and their risk of collapse was found to depend highly on the properties of their constituent materials.
Now, building on the success of their initial research, the Korean team have embraced a microfluidic approach to develop a second iteration of their technology, which is capable of producing lobule-like spheroids with a dramatically improved viability, that lends them potential within human cell-based therapeutic applications and the R&D of patient-specific medicines.
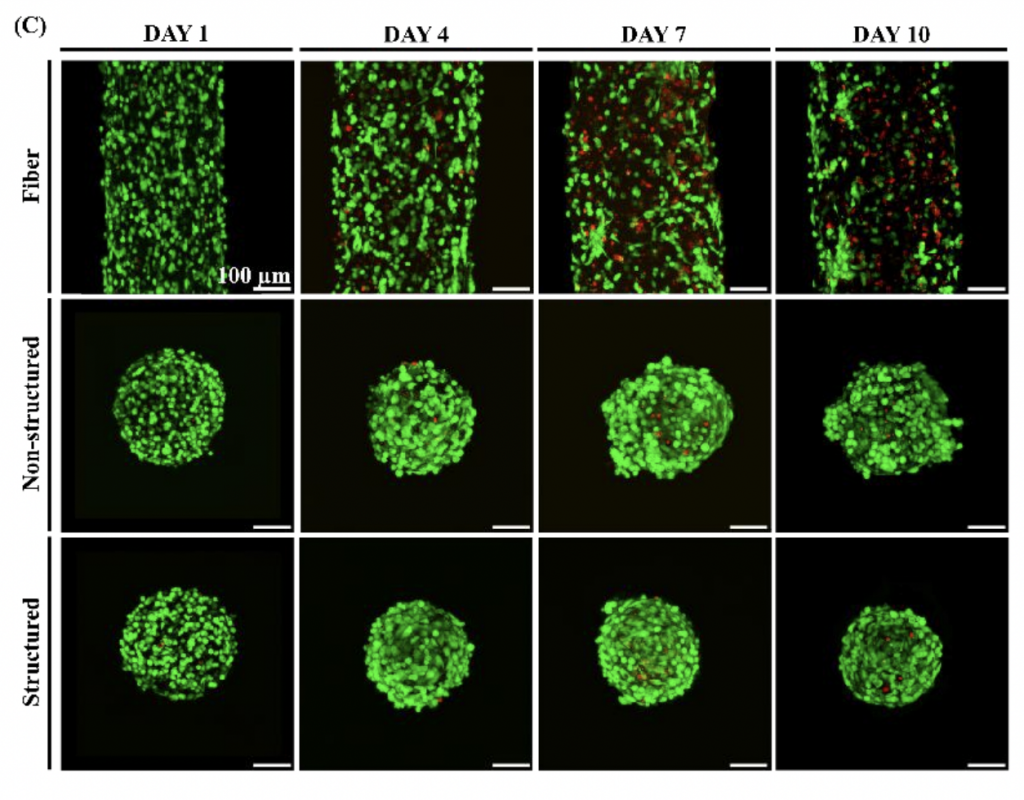
Liver tissue-transplant success
With their new technique, the T&R Biofab scientists have essentially combined extrusion with microfluidic emulsification, to produce spheroids at high-speed with a uniform size, without the need for crosslinking. In practise, the team’s approach saw them once again fill precursor cartridges with cells, but this time they deposited them into cross-sectional shapes, lending them a natural durability.
“The use of microfluidics enabled the generation of micro-liver spheres without the need of cutting the fiber into pieces,” explained Marinho. “This instantly brings high throughput drug screening, development and, luckily for us as we demonstrated in the paper, very high reproducibility without loss of viability in cells.”
During testing, the team assessed their improved methodology by producing several lobular microtissue spheroids, which compared to non-structured samples, each showed enhanced cell viability. What’s more, by four days after the initial experiment, the vascular structure had grown to a width of around 20 µm, a resolution that remains out of reach for many conventional extrusion bioprinters.
“Making a sphere as small as 250 micrometers with a highly compartmentalized structure that mimics the phenotype of in-vivo tissues is unheard of.”
Having demonstrated the efficacy of their technique, the researchers proceeded to test the implantation potential of their printed lobules by injecting them into lab mice. Not only did the lobular samples form blood vessels in-vivo, but their structural integrity improved significantly compared to non-structured alternatives, while experiencing nearly no loss of viability.
As a result, the T&R Biofab scientists believe that although their spheroids themselves aren’t novel, they possess a compartmentalized in-vivo structure that “is unheard of” in the industry, thus with sufficient R&D, the technique used to create them could be deployed to develop “revolutionary” treatments, which restore functionality to damaged human organs.
“We did not do clinical trials, so we cannot affirm that results are the same in humans,” concluded Marinho. “But, if any researcher wants to increase the chance of making tissue therapy a success, he/she should at the very minimum consider if his/her engineered tissues could be better, if they were phenotypically-similar to in-vivo tissues prior to transplantation.”
Organ 3D bioprinting’s potential
While techniques such as that being developed by T&R Biofab could eventually forgo the need for fully-printed organs altogether, multiple groups of researchers are currently working to achieve just that. For instance, through its Print to Perfusion program, 3D Systems is developing an ultra-precise means of 3D bioprinting fully-sized, vascularized human lung scaffolds.
In a similar vein, EPFL spin-out Readily3D has made significant progress in its work towards developing a 3D printed human pancreas model. Developed as part of the EU-funded Enlight project, the firm’s technology is reportedly capable of producing tissues within just 30 seconds for diabetes drug-testing applications.
Elsewhere, 3D bioprinting experts have remained split about its viability for creating transplantable organs, with the interaction between natural and synthetic tissues being identified as a potential issue. According to the Brain Research Institute’s Juan Carlos Marvizon, such organs can theoretically be programmed to prevent rejection from their hosts, although this hasn’t yet been tested in practise.
The researchers’ findings are detailed in their paper titled “Production of Multiple Cell-Laden Microtissue Spheroids with Biomimetic Hepatic Lobule-like Structure.”
The study was co-authored by Gyusik Hong, Jin Kim, Hyeongkwon Oh, Seokhwan Yun, Chul Min Kim, Yun-Mi Jeong, Won-Soo Yun, Jin-Hyung Shim, Ilho Jang, C-yoon Kim and Songwan Jin.
The nominations for the 2021 3D Printing Industry Awards are now open. Who do you think should make the shortlists for this year’s show? Have your say now.
To stay up to date with the latest 3D printing news, don’t forget to subscribe to the 3D Printing Industry newsletter or follow us on Twitter or liking our page on Facebook.
For a deeper-dive into additive manufacturing, you can now subscribe to our Youtube channel, featuring discussion, de-briefs and shots of 3D printing in-action.
Are you looking for a job in the additive manufacturing industry? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured image shows lab mice with both structured and unstructured 3D bioprinted lobular implants. Photo via the Advanced Materials journal.


