New research conducted by Korea Polytechnic University, and Gachon University in South Korea, demonstrates the use of a micro 3D printing technique for tissue engineering research. As a proof of concept, the researchers used Blu-Ray powered micro-stereolithography (MSTL) to make scaffolds suitable for the regeneration of bone cells.
The Blu-Ray MSTL process
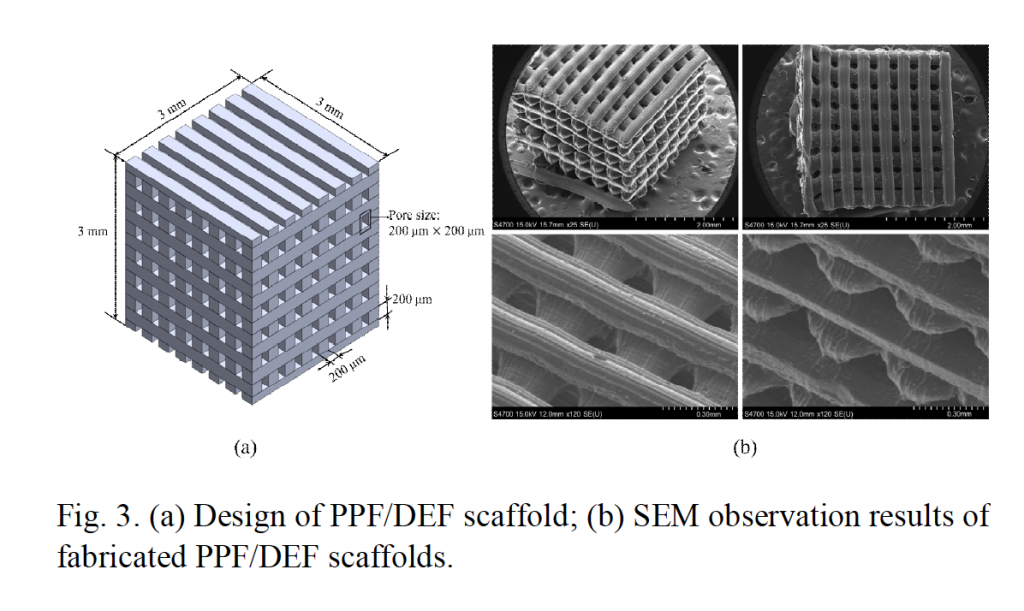
MSTL is a high resolution 3D printing technique capable of making objects “that have feature sizes of tens to hundreds of micrometers.” To put this in perspective, these features can amount to sizes smaller than the diameter of a human hair, which typically measures around 50 micrometers (microns, μm). This resolution is ideal for application to cell scaffolds – the structures that support the growth of living tissue – as they replicate the microstructure of cells as they occur in the body.
A form of stereolithography, MSTL 3D printing uses a projector to cure materials into a solid object. In this research, the projector is powered using Blu-Ray technology.
Reducing the cost of bone surgery
The microscopic scaffolds, measuring just 4 × 4 × 4 mm with a pore size of 200 × 200 μm, are MSTL 3D printed in a biodegradable photopolymer. After post-curing the scaffolds are laced with stem cells that culture to form bone marrow.
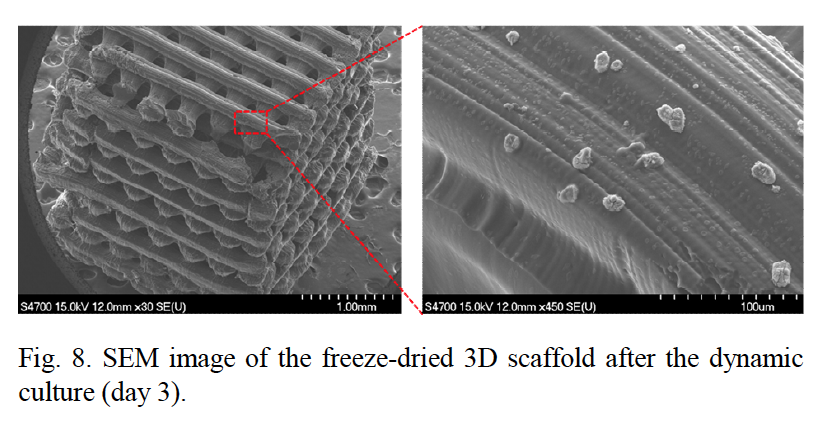
The seeded scaffolds are placed in a bio-reactor for the growth and multiplication of the cells. Conditions inside the bioreactor mimic blood flow, and stimulate cell proliferation through the introduction of a magnetic field. By comparison to ‘static’ scaffold samples outside of the reactor, cells exposed to the magnetic field exhibited a much higher rate of proliferation after 1, 3 and 7 days of incubation.
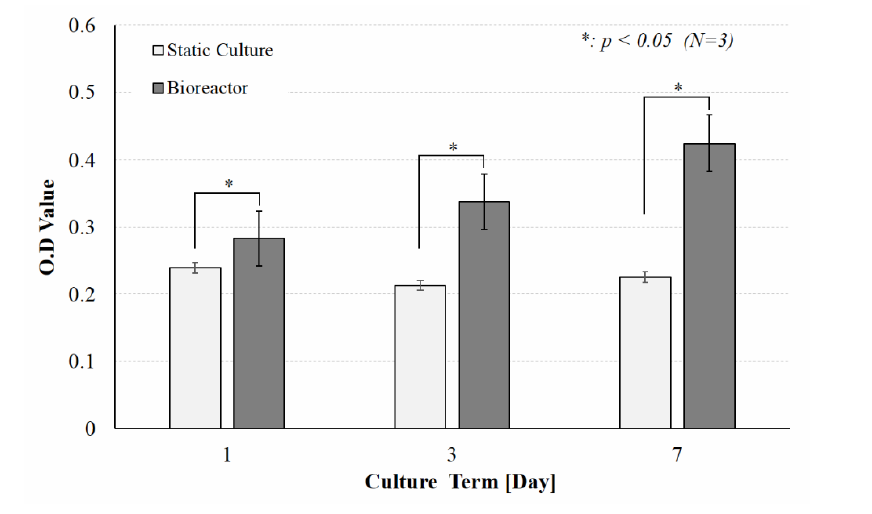
By accelerating this rate, the process demonstrates the “potential to reduce the cost of surgery for bone replacement”, as it cuts down on time taken to create bone tissue, and consequently reduces the risk of failure and loss of the samples.
The skeletons of regenerated organs and tissue
With the variety of tissue types in the body, e.g. muscle, bone, cartilage, there are many different approaches to creating scaffolds conducive to cell growth. 3D printing is widely used for such studies as it allows a design freedom impossible with any other method.
In a recent study from Tianjin University, the Chinese Academy of Sciences and the University of Hong Kong, researchers created a clay-based material, 3D printed and proven to cause accelerated regeneration of tibia defects in rats. Another approach, from Case Western Reserve University, uses graphene fused PLA to 3D print stronger bone-sportive scaffolds.
Fabrication and tissue engineering application of a 3D PPF/DEF scaffold using Blu-ray based 3D printing system discussed in this article is published online in the Journal of Mechanical Science and Technology Volume 31, Issue 5. It is co-authored by Jae-Hun Kim and Won-Soo Yun from the Department of Mechanical System Engineering at Korea Polytechnic, and Jin Woo Lee of the Department of Molecular Medicine at Gachon.
For more of the latest 3D printing research in medical, engineering and other applications sign up to the 3D Printing Industry newsletter, like us on Facebook and follow us on Twitter.
Featured image: Scanning electron microscope (SEM) images of micro 3D printed scaffolds used to support cell growth. Image via Kim, Lee & Yun


