Researchers at the Munich University of Applied Sciences have developed a highly accurate 3D bioprinting approach that’s capable of creating human tissues at a single-cell resolution.
By firing an ultrashort Near Infrared (NIR) laser into a hydrogel positioned just underneath a layer of cells, the team have been able to eject tiny cellular clusters, and pack them into a 3D scaffold. The novel process allows individual cells to be transferred based on their morphology at a viability of 93-99%, and with further automation, the scientists believe that it could yield functional tissue transplants.
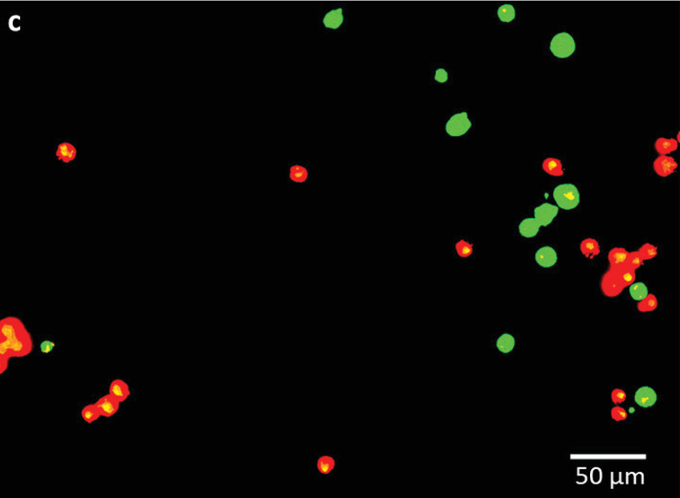
High-resolution 3D bioprinting
Within scientific circles, the potential of bioprinted soft tissue substitutes is well-known and much experimented-on, particularly when it comes to drug R&D and preventing age-related cellular degeneration. Despite this, and the fact that many different approaches to creating tissue scaffolds have been developed in recent years, precisely positioning individual cells remains a significant challenge.
Although inkjet‐based and drop‐on‐demand approaches have previously demonstrated single-cell precision, they often suffer from both low accuracy and viability. Alternatively, acoustic cell patterning techniques have proved less damaging and achieved high levels of resolution, but these methods don’t allow for the manipulation of specific cells.
Given the potential of pinpoint single-cell control in encouraging proliferation and ultimately designing tailored, functional human soft tissues, the scientists developed their novel approach in 2018. Based around a pulsating NIR laser, the team’s technique allowed them to transfer small hydrogel droplets of around 10-30 cells onto a target substrate.
In their latest research, the Munich scientists have now refined this procedure, to allow the selection of specific cells from a reservoir, before moving them to a target surface. With a high level of accuracy, resolution and cell survivability, the researchers’ revised technique could represent a significant step towards more viable tissue transplants.
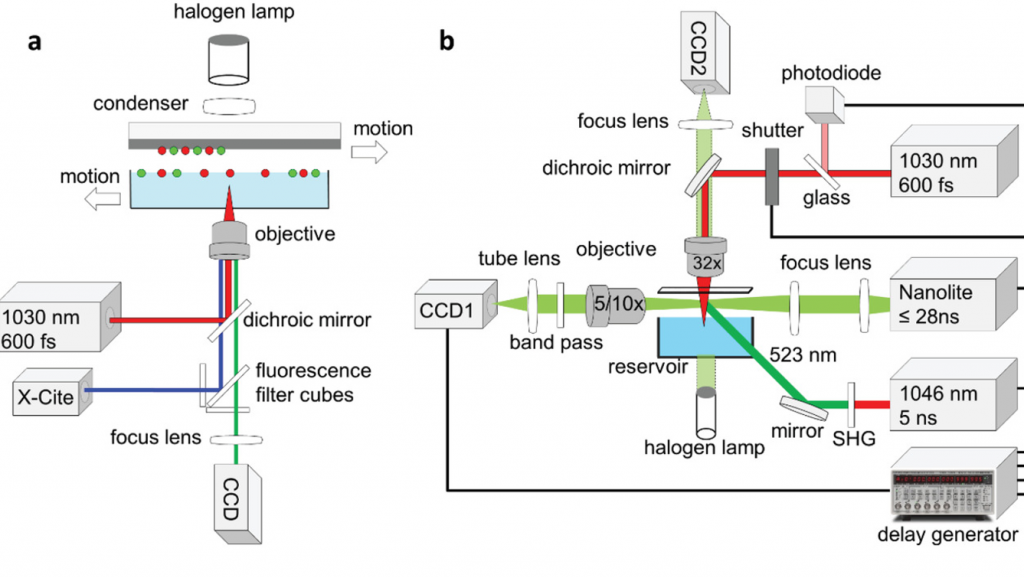
The novel Munich method
Within the researchers’ optimized approach, they mounted their original setup onto an inverted optical microscope, on which they could identify cells based on their shape, size or fluorescence. A 1030 nm laser was then fired into the underlying hydrogel, at a wavelength that was sufficient to cause cells to ‘jet’ away in either single or multiple groups, without damaging their overall viability.
During testing, the scientists printed rows of human mesenchymal stem cells (hMSCs) with gaps of 50, 100, and 200 µm onto a gelatin‐coated substrate. After detailed analysis, results showed that the team had achieved an extremely high level of accuracy, with most cells deviating from their target position by less than one cell diameter (14-32 µm).
To assess the impact of their technique on the viability of the hMSCs, the researchers then deposited them onto a collagen‐laden surface, achieving a survivability of up to 100%. However, cells spaced at a distance of 200 µm actually moved away from each other, suggesting that their mechanical signals couldn’t reach neighboring cells.
Additionally, manually transferring hMSCs was found to take around 20 seconds per cell, limiting the process’ scalability. In order to ramp-up cell transfer speeds, the scientists believe that in future, it could be possible to combine their process with two‐photon stereolithography (2PP), enabling the creation of novel organ-on-a-chip devices and eventually functional human tissue substitutes.
Mixed bioprinting approaches
Bioprinting remains an experimental but rapidly advancing technology, and as a result, a variety of cellular deposition approaches are currently in development.
Researchers from the University of Bath and the University of Bristol have developed a new acoustic-energy bioprinting technique called ‘sonolithography.’ The team’s novel process, which uses ultrasound waves to precisely deposit particle patterns onto a substrate, could be deployed in future within drug testing applications.
Scientists based at the University at Buffalo have taken a completely different approach, by devising a rapid vat-SLA-based 3D bioprinting process. Using their technique, the researchers were able to reduce the time it takes to create cell-laden hydrogel structures, from over six hours to just nineteen minutes.
By contrast, researchers such as those at the University of Montreal, have recently sought to adopt low-energy lasers as means of developing new ‘drop-on-demand’ 3D bioprinting methods. Broadly, the team’s approach involves jetting cells onto each other to form larger droplets, which could be used to build future soft tissues.
The researchers’ findings are detailed in their paper titled “Single Cell Bioprinting with Ultrashort Laser Pulses.” The research was co-authored by Jun Zhang, Patrick Byers, Amelie Erben, Christine Frank, Levin Schulte‐Spechtel, Michael Heymann, Denitsa Docheva, Heinz P. Huber, Stefanie Sudhop and Hauke Clausen‐Schaumann.
To stay up to date with the latest 3D printing news, don’t forget to subscribe to the 3D Printing Industry newsletter or follow us on Twitter or liking our page on Facebook.
Are you looking for a job in the additive manufacturing industry? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured image shows the scientists’ luminescent-labelled human stem cells. Image via the Advanced Functional Materials journal.


