Digital services firm Ricoh USA has received 510(k) clearance from the US Food and Drug Administration (FDA) for its Ricoh 3D for Healthcare workflow.
The workflow has been cleared for craniomaxillofacial and orthopedic patient-specific anatomic modeling, which the company sees as the next major step in providing widespread access to patient-specific 3D models for healthcare providers.
“Ricoh is committed to healthcare innovation that will turn the tide on patient engagement and precision medicine,” said Gary Turner, Managing Director, Additive Manufacturing, North America, at Ricoh USA. “Ricoh 3D for Healthcare does just that – offering a model matched to the unique anatomy of each individual patient.
“One of our goals is to address the needs of doctors and patients in specific areas with particularly high demand for 3D printed anatomic models, the 510(k) clearance for craniomaxillofacial and orthopedic models moves us further toward reaching this goal.”

3D printing anatomical models
Additive manufacturing has been deployed for the design and production of anatomical models in the healthcare sector for some time, and is one of the more established applications of the technology.
Combined with 3D scanning and X-rays, 3D printed models can provide a far more accessible, customizable, and low-cost alternative to cadavers in aiding surgeons in better preparing for surgeries. In turn, this can improve treatment success rates and patient outcomes.
As 3D printing technologies have improved, various firms have produced anatomical models with previously unattainable degrees of color fidelity and achieved significant surgical time and cost savings. Additionally, a growing number of companies are gaining ISO certification and FDA clearance for their 3D printed models.
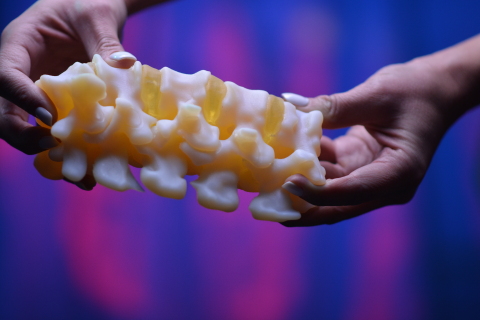
Ricoh 3D for Healthcare
Ricoh 3D for Healthcare is an end-to-end workflow created to simplify the design, development, and production of 3D printed anatomical models. The workflow was developed in partnership with IBM Watson Health, the healthcare arm of technology consultancy IBM, and makes use of the firm’s IBM iConnect Access diagnostic and image exchange platform.
The two companies teamed up to integrate a 3D printing feature into the platform to enable IBM iConnect Access users to 3D print lifelike anatomical models using patient medical imaging data directly from the platform.
In November, Ricoh USA partnered with industrial 3D printer manufacturer Stratasys to integrate the latter’s J750 Digital Anatomy and J5 MediJet 3D printers into the Ricoh 3D for Healthcare workflow.
The platform is offered through two different avenues, the first is a point-of-care option where Stratasys’ machines are paired with Ricoh USA’s managed services staff on-site to oversee the printing process. The second is an on-demand service through which providers can order anatomical models to be 3D printed and shipped directly to them.
Through their partnership and the Ricoh 3D for Healthcare workflow, Ricoh USA, Stratasys, and IBM Watson Health are hoping to overcome many of the barriers facing healthcare providers looking to run their own 3D printing facility, such as budget and staffing constraints, training requirements, and HIPAA and quality compliance.
Now that the workflow has received 510(k) clearance from the FDA, Ricoh 3D has taken the next step toward providing healthcare providers with access to patient-specific 3D models. As a result, healthcare providers and clinics will be able to deliver precision medicine and improve patient and surgical outcomes.
Going forwards, Ricoh USA is planning to solicit 510(k) clearance for additional treatment fields.
Subscribe to the 3D Printing Industry newsletter for the latest news in additive manufacturing. You can also stay connected by following us on Twitter and liking us on Facebook.
Looking for a career in additive manufacturing? Visit 3D Printing Jobs for a selection of roles in the industry.
Subscribe to our YouTube channel for the latest 3D printing video shorts, reviews, and webinar replays.
Featured image shows a 3D printed anatomic model via the Ricoh USA for Healthcare workflow. Photo via Ricoh USA.


