Researchers from the University of Oregon (UoM) have developed a 3D printed device that’s capable of recording and stimulating the electrical impulses that drive vocalizations within songbirds.
The team’s microscopic device, featuring a tiny thin-film electrode with a 3D printed housing, functions by decoding and modulating electrical signals sent to either the brain or spinal cord. Leveraging their novel ‘nanoclip,’ the scientists were able to precisely control nerve output within a number of zebra finch test subjects, evoking distinct predetermined vocalizations.
In the future, the technology behind the tiny device could be deployed within keyhole surgical applications, or even in the creation of new bioelectronic medicines for diseases such as diabetes or arthritis.
“Imagine you had to manipulate a small nerve and wrangle a device onto it using forceps to both open a cuff electrode and position it on the nerve,” said Tim Gardner, a neuroscientist at the University of Oregon. “The micromanipulation required with current cuff electrodes could be damaging to the smallest nerves. In contrast, the 3D-fabricated nanoclip can be implanted by just pushing it onto the nerve.”
The therapeutic benefits of nerve stimulation
An increasing number of clinical trials are showing that the targeted use of electrical modulation within the Peripheral Nervous System (PNS) can have positive therapeutic effects. Early studies have demonstrated that nerve stimulation can effectively treat a range of illnesses such as inflammatory diseases, depression, and epilepsy, amongst others.
In order to harness the potential of these bioelectronic therapies, new electrode-based devices need to be developed, that are capable of both mapping and controlling nerve behavior. These new Peripheral Nerve Interface (PNI) technologies will require scalability, flexibility, and the ability to be tailored to individual patients if they are ever to find end-uses within a clinical setting.
Previous PNIs can broadly be categorized as intraneural, regenerative, or extra-neural devices. Intraneural PNIs can often be placed in very close proximity to the nerve, yielding excellent recording and stimulation abilities. On the downside, such devices are usually quite large, which causes a substantial risk of scarring, nerve trauma, and interrupted blood flow.
“Future devices will involve a combination of thin-film microfabrication and 3D printing on a micron scale.”
By contrast, regenerative PNIs exploit the ability of peripheral nerves to re-establish connections after transection, but this approach requires lengthy periods of waiting for nerves to regrow. As a result, regenerative solutions have found limited therapeutic uses, and they have generally been difficult to experiment with. Extra-neural electrodes are the most widely-used form of PNI, because they use an insulating polymer to avoid any nerve damage at all.
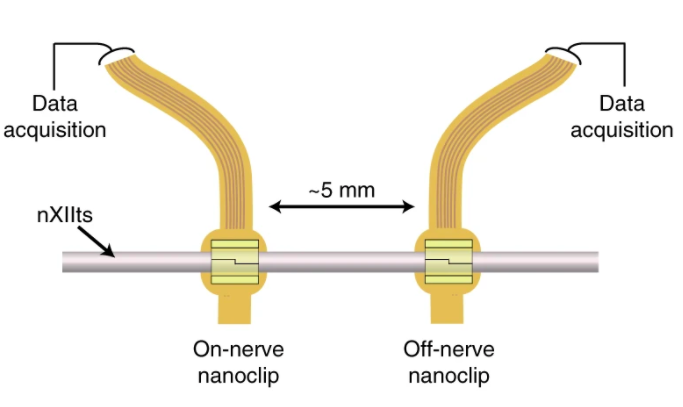
The smaller nature of extra-neural PNIs comes at a cost though, and their reduced electrical access to nerve fibers causes them to have limited recording and stimulating capabilities. Attempting to optimize the extra-neural approach, the Oregon team integrated a more powerful multichannel thin-film electrode into the nanoclip design they initially developed back in 2017.
Utilizing their upgraded device, the team hypothesized that a high level of Signal-to-Noise Ratio (SNR) recordings could be achieved. More substantially, the researchers also believed that precise modulation would enable them to control organ functionality. The technology behind such nerve stimulation could be potentially lucrative, unlocking a spate of enhanced therapeutic treatments in the future.
The Oregon researchers’ 3D printed stimulator
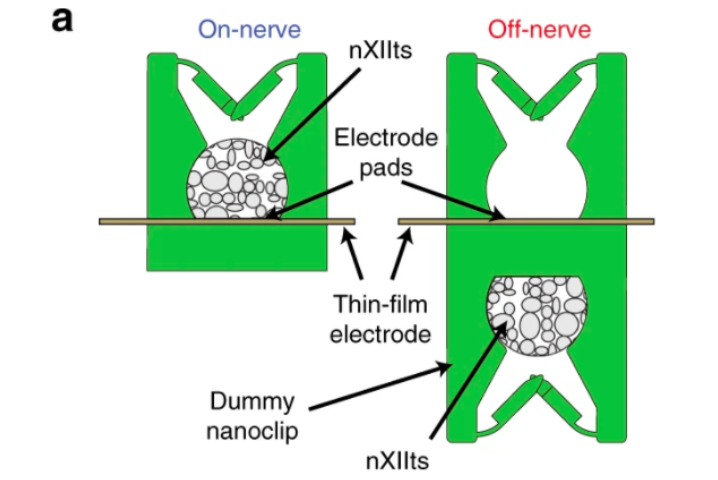
In order to create their microstimulation device, the scientists deployed a combined thin-film microfabrication and nanoscale 3D printing approach. Matching the scale and peripheral tissue of small nerves, the thin-film electrode array was produced with a 50 nm layer of gold, and an overall depth of 12 µm and a narrow width of 250 µm.
The electrode array itself consisted of six gold pads (45 × 80 µm each in a 2×3 grid), and this was placed within a 260 × 105 µm region on the probe’s head, giving it a total length of 40 mm. To secure the device onto the nerve, the team used their 3D printed nanoclip, which consisted of two hinged trap doors at the entrance of a semi-cylindrical cavity, that passed right through the array and held it in place.
Leveraging an additive manufacturing-based approach enabled the team to quickly fabricate the stimulator in a precise and repeatable way, with a cheap and freely-available thermoplastic. Additionally, by modelling the device beforehand, the scientists were able to reduce potential errors that could cause irritation within potential future patients.
To test the efficacy of their nanoclip device, the team performed finite element mechanical modeling of its trap doors. Results showed that its impact on the nerve would have an upper force limit of ~120 µN, significantly more than the 1.25–7.5 µN required. Simulation analysis also revealed that the device was sufficiently robust to withstand the biomechanical forces of implantation and body dynamics, another hurdle preventing the adoption of other PNIs.
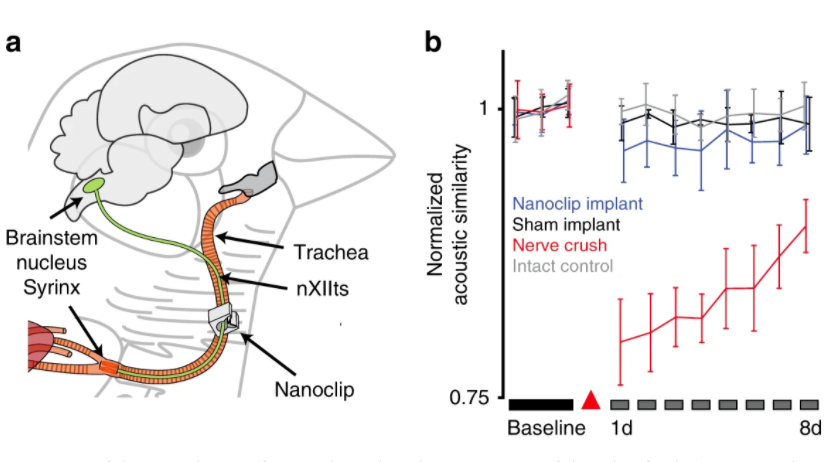
Aiming to validate the in vivo abilities of their nanoclip, the Oregon team stimulated and recorded compound responses from the tracheosyringeal nerves of anesthetized zebra finches. The songbirds’ nerves were used because they are similar to those exhibited in other mammales, and nerve disruption caused by the device would lead to an audible change in the finches’ repetitive song.
Following implantation, the researchers were able to evoke compound responses from the birds, while only causing a small and insignificant change to their more accustomed birdsongs. Following the success of their initial trials, the team stated that the microscale 3D printing technology behind their microclip device, could potentially hold a range of human applications in future.
“This study is really an early test for new fabrication methods focused on submillimeter structures,” Gardner said. “The main focus of the work in my lab is to refine methods for integrating thin-film fabrication and micron-resolution 3D printing, and to use these tools to create new kinds of devices.”
The wide-ranging applications of nano 3D printing
Although several companies have already commercialized nanoscale additive manufacturing, the technology itself is still very much in the early stages of development. As a result, a number of researchers are currently working to optimize the nano-production process.
Researchers from the University of Dayton have developed an enhanced and more cost-effective method of 3D printing nanoscale structures. The Opto-Thermo-Mechanical (OTM) nano-printing technique proved capable of printing at an accuracy level of fewer than 100 nanometers.
Scientists from the Fraunhofer Institute for Microengineering and Microsystems (IMM) are working on a novel process for fabricating nanostructures. By combining two-photon absorption with commercially-available alloys, the team were able to 3D print in nanoscale directly onto metal parts.
A research team at the Department of Energy’s Oak Ridge National Laboratory have used Focused Electron Beam Induced Deposition to 3D print nanoscale structures. Working with a team from the Graz University of Technology, they developed a simulation-based technique for creating high-fidelity nano-objects predictably and repeatedly.
The researchers’ findings are detailed in their paper titled “Printable microscale interfaces for long-term peripheral nerve mapping and precision control,” which was published in the Nature Communications journal. The report was co-authored by Timothy M. Otchy, Christos Michas, Blaire Lee, Krithi Gopalan, Vidisha Nerurkar, Jeremy Gleick, Dawit Semu, Louis Darkwa, Bradley J. Holinski, Daniel J. Chew, Alice E. White and Timothy J. Gardner.
Nominations for the 2020 3D Printing Industry Awards are still open, let us know who is leading the industry now.
The fourth edition of the 3D Printing Industry Awards Trophy Design Competition is now underway. Enter your design for the chance to win a CraftBot Flow 3D printer.
To stay up to date with the latest 3D printing news, don’t forget to subscribe to the 3D Printing Industry newsletter or follow us on Twitter or liking our page on Facebook.
Are you looking for a job in the additive manufacturing industry? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured image shows an illustration of neurons in the human nervous system. Image via UoM.


