A joint research team from the Korea Advanced Institute of Science and Technology (KAIST) and the Korea Institute of Science and Technology (KIST) has developed an innovative hydrogen production catalyst which it claims is 20 times more efficient than others currently in existence.
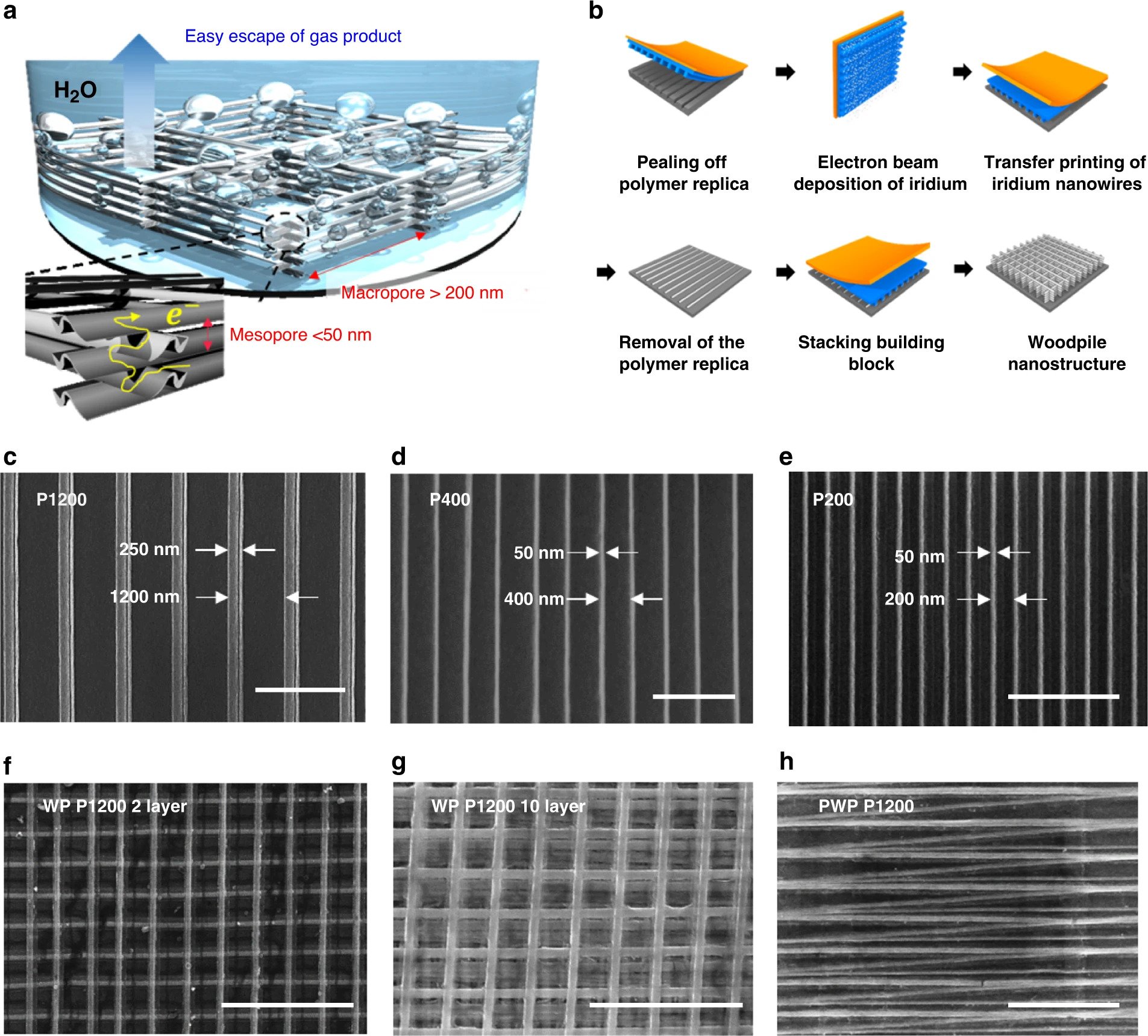
The researchers constructed the catalyst through solvent-assisted nanotransfer printing (S-nTP), a technique very similar to 3D printing, which involves stacking Iridium nanowire arrays in the shape of a ‘woodpile’ structure. This uniform structure allows the hydrogen gas bubbles generated by the catalyst to escape far more efficiently than in conventional iridium nanoparticle catalysts with random arrangements.
The team believes this newly developed technology could significantly reduce the use of expensive iridium catalysts, which are valued the same as gold, and provide a more cost-effective method of producing hydrogen.
Hydrogen production catalysts
As the world looks to shift from fossil fuel-centered energy supply to more renewable options, many deem the hydrogen economy a next-generation industrial structure with substantial potential. Considered eco-friendly and highly efficient, hydrogen is, however, expensive to produce, with the issue of cheaply producing it considered one of the key barriers to its wider take-up.
Polymer Electrolyte Membrane Water Electrolyzers (PEMWEs) produce high-purity hydrogen through electrolyzing water using solar cells or surplus electric energy, and have attracted strong interest due to their compactness, high energy efficiency, hydrogen purity, and efficient production rate. The limitation for large-scale industrialization of PEMWEs is the requirement of expensive metal catalysts, such as Iridium, to facilitate the oxygen evolution reaction (OER) which produces the hydrogen.
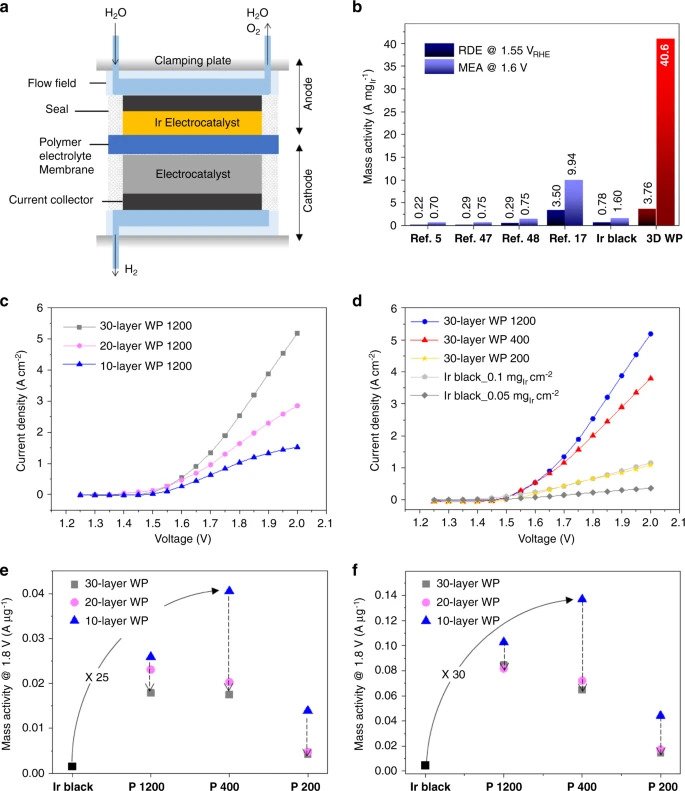
Significantly, the mass activity of these OER catalysts is determined by the electrochemically active surface area per loaded mass of Iridium (ECSA) and mass activity per ECSA. The KAIST and KIST team, therefore, saw an opportunity to re-engineer the catalyst’s nanostructures in order to improve its overall efficiency by reducing the amount of Iridium used, which in turn substantially lowers costs.
Nanotransfer printing
The nTP method has received considerable attention thanks to its ability to readily generate highly functional nanostructures with 2D and 3D arrangements in a low-cost and highly efficient way. During the process, nanostructures are generated through vapor-phase or solution-phase deposition of material onto an elastomeric mold and are then transferred onto other substrates using a direct contact mechanism.
Through KAIST and KIST’s ultra-high-resolution S-nTP process, highly ordered nanowire arrays were repeatedly printed via electron beam deposition and transferred onto a macroscopic area in a multilayer stack, which once completed eventually formed a woodpile-structured Iridium electrocatalyst.
Further detail on S-nTP can be found in a previous paper by the researchers titled “High-resolution nanotransfer printing applicable to diverse surfaces via interface-targeted adhesion switching”, published in the Nature Communications journal. The article was co-authored by J. Jeong, S. Yang, Y. Hur, S. Kim, K. Baek, S. Yim, H. Jang, J. Park, S. Lee, C. Park, and Y. Jung.
KAIST and KIST’s findings
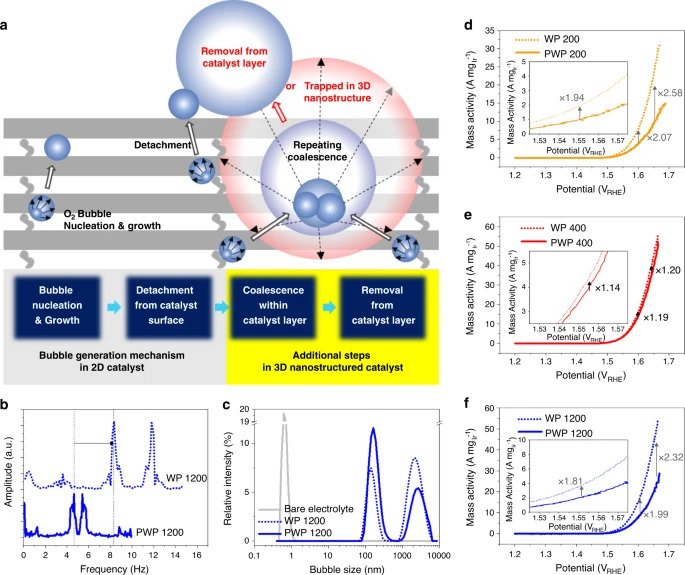
Through their S-nTP process, the research team was able to reveal significant improvements in ECSA activity resulting from the uniform access of water reactants to the catalytic surface. The study also revealed the method enabled the easy transport and removal of evolved oxygen gas products through the catalyst’s well-defined pore channels, optimized through the re-engineering of the catalyst’s nanostructures.
As a result, the woodpile-structured catalyst was proven 20 times more efficient than other hydrogen production iridium catalysts currently in existence.
In the article, the researchers concluded: “We expect that these outcomes will provide a large step toward the development of highly feasible OER catalysts by simply controlling their 3D microstructures even without any change of physiochemical characteristics. Therefore, there will be abundant room for further improving the OER performance of our woodpile-structured catalysts via synergistic combination with other approaches on new elemental compositions.”
Further details of the study can be found in the article titled “Highly efficient oxygen evolution reaction via facile bubble transport realized by three-dimensionally stack-printed catalysts,” published in the Nature Communications journal. The study is co-authored by Y. Kim, A. Lim, J. Kim, D. Lim, K. Chae, E. Cho, H. Han, K. Jeon, M. Kim, G. H. Lee, G. R. Lee, H. Ahn, H. Park, H. Kim, J. Y. Kim, and Y. Jung.
Subscribe to the 3D Printing Industry newsletter for the latest news in additive manufacturing. You can also stay connected by following us on Twitter and liking us on Facebook.
Looking for a career in additive manufacturing? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured image shows the fabrication of 3D Woodpile-structured Iridium catalysts via nanotransfer printing. Image via Nature Communications journal.


