Dr. Burcin Ekser and his research team at Indiana University School of Medicine (IUSM) have been awarded $9 million to further their 3D bioprinting research for the development of transplantable organs.
A three year deal, the money was contributed by Lung Biotechnology PBC, a wholly-owned subsidiary of United Therapeutics Corporation (NASDAQ: UTHR), that is also working with 3D Systems.
At the time of the funding announcement, Dr. Esker commented, “This alliance with Lung Biotechnology will greatly enhance our ability to accomplish our ultimate goal of providing an unlimited supply of organs to save human lives.”
Highlighting the transplantable organ shortage, Dr. Esker added:
“It’s my passion because I’m a transplant surgeon; I don’t want anyone to die while they’re waiting for a transplantable organ.”
An alternative to animal testing?
Dr. Ekser and the team are responsible for IUSM’s xenotransplantation lab. Xenotransplantation is the practice of cross-species transplantation and, due to close biological similarities, the team is working develop ways of producing pig organs that can be donated to humans.
In one area of interest, the IUSM team is working with CRISPR gene editing to bioengineer pig organs that are even more suited to human transplant.
In a second area, the team is investigating how to use 3D bioprinting to grow organs from a sample of pig cells, eradicating the use of live animal.
Put the needle on it
3D bioprinting technology used at IUSM is the Kenzan method, marketed in the Regenova 3D bioprinter from Cyfuse Biomedical K.K. A “scaffold-free” approach, the Kenzan method is based on a needle array, tightly arrange into a square.
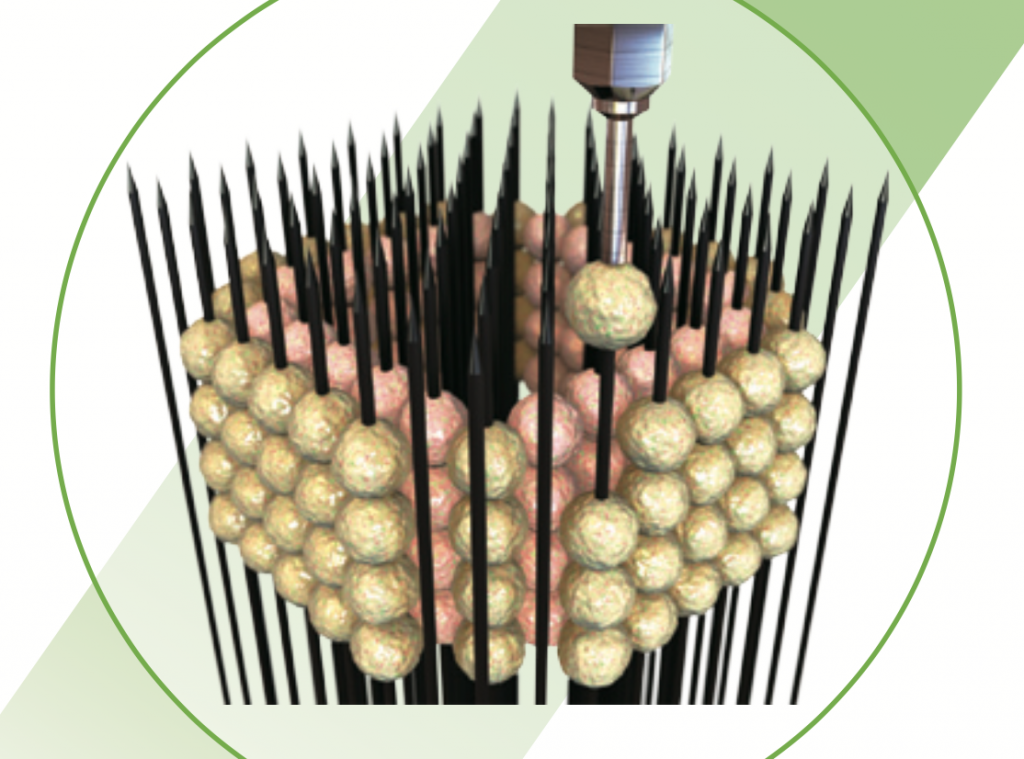
To 3D print, lab-grown cells are individually skewered on to each needle in the array, gradually building up a desired shape, e.g. a cylindrical vessel.
Neatly packed against each other, these cells naturally fuse together when left to culture, creating a complete tissue that can be removed from the needles.
Left to culture for a second time, these cells grow to fill the voids left by needle pricks, giving a complete living tissue sample.
Next, in IUSM’s work, the tissue sample is kept inside a specially designed, and 3D printed bioreactor called FABRICA. With FABRICA, the scientists can replicate blood flow through the 3D bioprinted tissue and monitor its reaction.
At the University of Tokyo, the same method has been used before to 3D bioprint a “mini-liver” tissue sample.
Five day mini-organs
With Kenzan 3D bioprinting, the IUSM team have proven the ability to “[3D] print the miniature pig organ models within a day, grow and mature them within five days” according to Dr. Ekser, then test them over the course of two weeks to achieve a desired outcome. A great deal of blue-sky thinking goes into the effort at the xenotransplantation lab, and Dr. Ekser confirms the vision: “So people go to the hospital in the future, and we will print the organ you need,”
“Then we basically will not have any shortage of organs.”
Through the collaboration with Lung Biotechnology PBC the lab’s aim “is to advance the field, so we can reach that goal as soon as possible.”

Lung Biotechnology PBC
Lung Biotechnology PBC was founded in 1997 to conduct research and development into ways to preserve and assess lungs for transplant. As an extension of United Therapeutics, it is also involved with the clinical trials of new drugs to treat heart disease. Adcirca, Orenitram, Remodulin and Tyvaso are some of the drugs already on the market from its parent company. And Beraprost 3, meanwhile, is a new synthesis that has been in trials since 2013.
In April 2017, 3D Systems announced its plans to collaborate with Lung Biotechnology PBC on developing 3D bioprinting solutions.
At the time of the announcement, 3D Systems CEO Vyomesh Joshi, said, “We believe bioprinting is a powerful opportunity and we are uniquely positioned with the broadest portfolio of technologies to partner with companies of the caliber of United Therapeutics to provide healthcare solutions of the future.”
For more 3D bioprinting partnership updates subscribe to the 3D Printing Industry newsletter, following us on Facebook and liking us on Twitter.
For 3D Printing Jobs, sign up here.
Featured image shows from left to right: Lester Smith, PhD; Burcin Ekser, MD, PhD; and Ping Li, PhD of the University of Indiana’s Xenotransplantation lab. Photo by Erich Schoch/IU


