3D bioprinting, microfluidics and hydrogel scaffolding combine in the latest liver tissue research by a team of scientists attributable to seven institutions from across the globe.
Centralized at Harvard Medical School and the Harvard-MIT Division of Health Sciences and Technology at Massachusetts Institute of Technology (MIT), the organ-on-a-chip device seeks to improve the way drugs are tested.
It serves as an example of how drug testing can be safer, more reliable, and kinder to animals and to humans.
The 1 in 5,000 success rate
According to figures published by the New York Times in 2015, only one in every 5,000 new compounds makes it through classification to become an approved drug.
In typical methodology, single-layer petri dish samples or animals are used to reach the human clinical stage of drug testing.
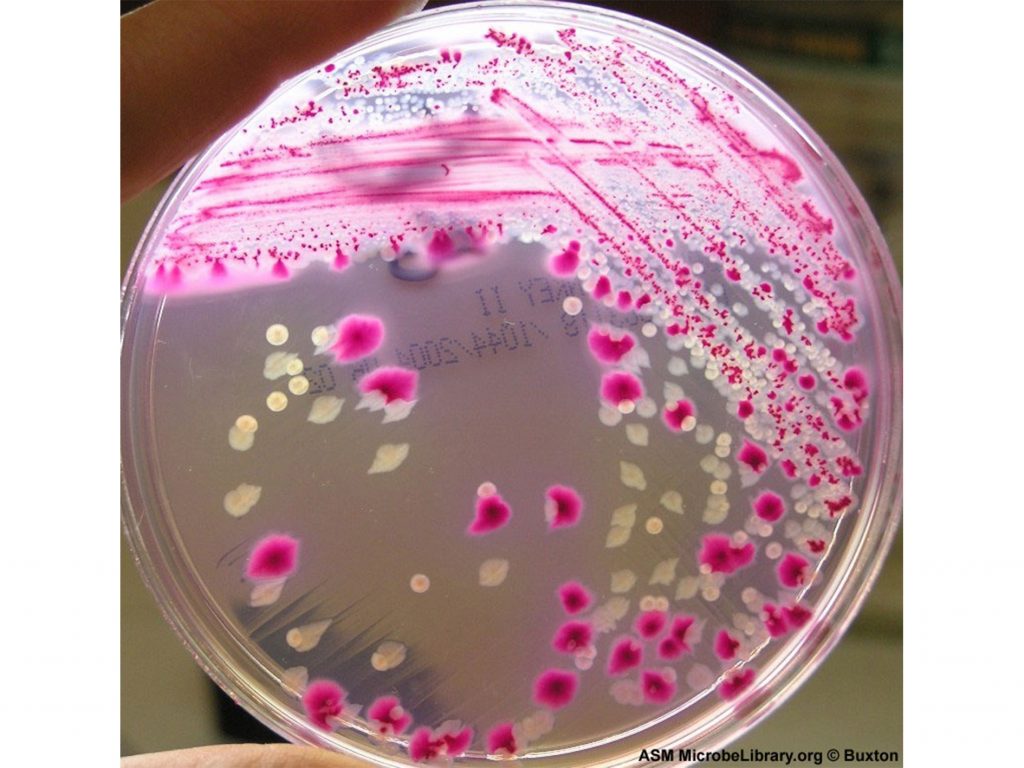
Ultimately both methods fail to represent the variety of tissues within the body. Animal testing also has the added implication of cruelty, and has been outlawed throughout Europe.
A liver written in jelly
In place of the traditional methods, the approach led by Ali Khademhosseini and Su Ryon Shin makes a 3D tissue model from living human cells.
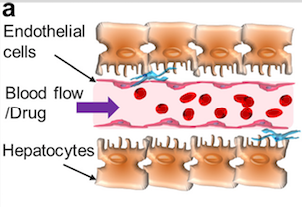
As in previous organ-on-a-chip research, the goal of the liver model is to create tissue matching the structure of a vessel, i.e. the base structure of all muscles.
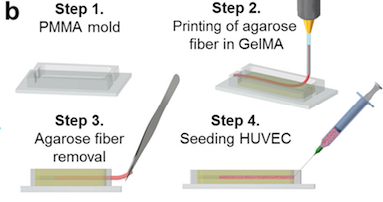
First, human liver cells (HepG2/C3A) are combined with a gel to make a structurally supportive mold.
The shape of vessels, as arranged in the liver, is written into this jelly in an algae-based ink.

The algae ink is dissolved, leaving a hollow channel in the center of the gel.
Cell sorting out
Using a syringe, umbilical vein endothelial cells (HUVECs) are pumped into the hollow channel and take on the shape of a vessel. When blood flow is introduced, the class perform the process of “sorting out”, becoming fully vascularised.
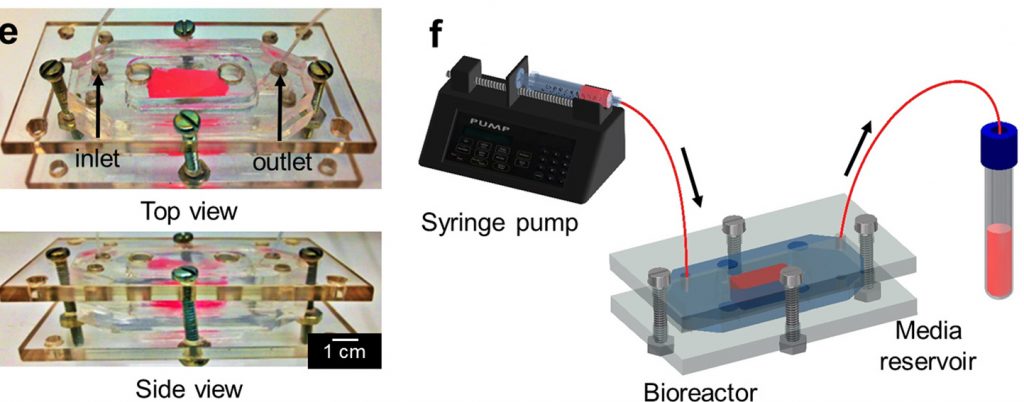
This microfluidic chip is then kept inside a bioreactor, maintaining constant conditions for cell growth and proliferation.
“Narrowing the gap”
Conclusions show that “The integration of our bioprinted vascularized construct with a bioreactor contributes to the creation of a more realistic “liver-on-a-chip” platform narrowing the gap between in vitro and in vivo drug testing models.”
The researchers add, “By creating advanced in vitro platforms that aim to replicate biological functions and environments, this vascularized platform will help observe and predict drug toxicity mechanisms at a microcirculatory level.”
To read more 3D printing related research daily follow us Twitter, like us on Facebook and sign up to our newsletter here.
For the latest activities in your areas, check out our 3D printing events page.
Featured image: Repeating image of false coloured 3D printed cells used to make a microfluidic liver chip. Original image via Shin et al.


