The U.S Food and Drug Administration (FDA) has awarded grants to five research institutes for the study and improvement of biomanufacturing, including 3D bioprinting.
Scott Gottlieb, MD, FDA Commissioner, said, “Advanced manufacturing technologies, such as continuous manufacturing, hold great promise for improvements in the reliability, flexibility, and cost-effectiveness of manufacturing for biological products.”
“we awarded in grants to five globally-recognized research institutions to study ways in which cutting-edge manufacturing technologies can be implemented to advance these goals, and enable more innovative, consistent, and dependable manufacturing of biological products”
Each research institute will focus on a specific area in the field of biomedicine. The five institutes granted the award are:
– Harvard University, Cambridge, Massachusetts, $599,910
– Carnegie-Mellon University, Pittsburgh, Pennsylvania, $599,844
– Rutgers University, Piscataway, New Jersey, $600,000
– Georgia Institute of Technology, Atlanta, Georgia, $600,000
– Massachusetts Institute of Technology, Cambridge, Massachusetts, $600,000
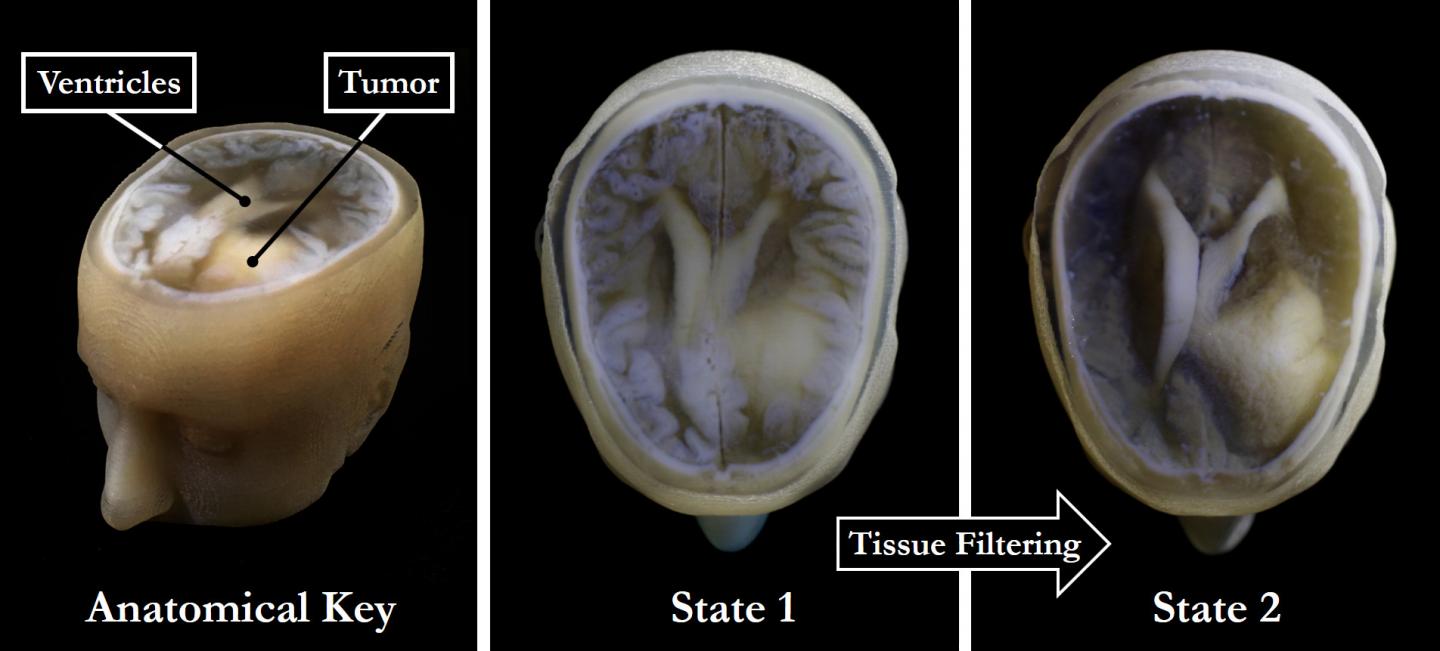
Carnegie-Mellon University will focus its assets on “addressing key manufacturing needs for extracellular matrix scaffold 3D printing.”
21st Century Cures Act
The FDA invoked the 21st Century Cures Act (Cures Act) to award the five grants. The Cures Act (December 13, 2016) gives the federal agency the authority to develop projects that would accelerate innovations in medicine.
Such projects include the Regenerative Medicine Advanced Therapy (RMAT), and the Breakthrough Devices program.
RMAT is an expedited option for eligible biological products. These products include regenerative medicine therapy drugs, those intended to treat life-threatening illnesses, and drugs which have “potential to address unmet medical needs” for life-threatening illnesses.
The Breakthrough Devices program speeds up the review process of certain medical devices. For a medical device to be considered under this program it is necessary to prove:
– the device is a more effective treatment of life-threatening condition than treatments currently available
– the device is a breakthrough technology and no approved alternative is marketed in the U.S
– the device is an improved solution over existing alternatives
– The availability of the device is in the best interest of patients
FDA and 3D printing technology
The FDA has been actively supporting the adoption of 3D printing technology in the biomedical and dental industry. Last year the agency made a statement regarding its commitment to the technology and released guidelines for manufacturers submitting 3D printed implants and other medical devices.
Many 3D printing companies responded positively to the FDA guidelines. Since then there has been a surge in 3D printed medical devices and their approval by the FDA.
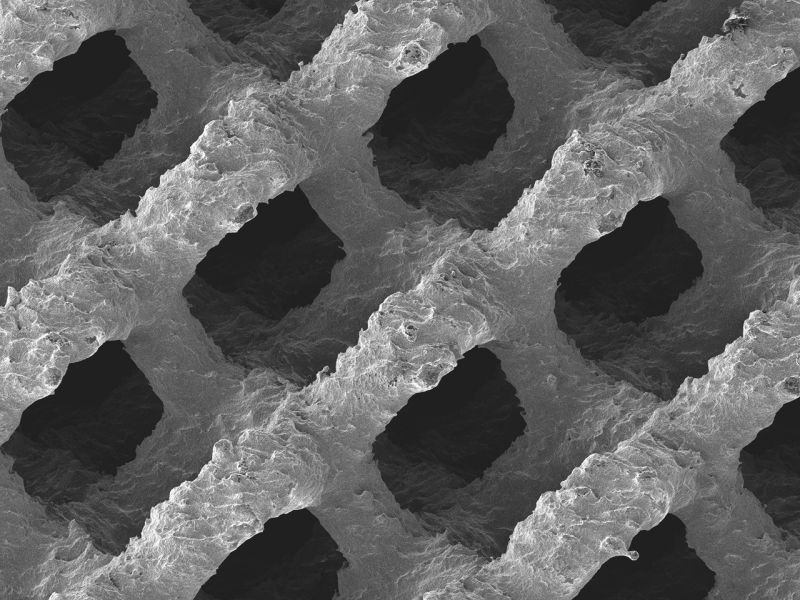
In 2017, SI-BONE, the manufacturer surgical implants, announced FDA clearance for its patented iFuse Implant System, a 3D printed titanium implant for SI joint. The same year, 3D printed Cellular Titanium spinal support implants by Emerging Implant Technologies, a German spinal implants manufacturer, were approved by the FDA.
Earlier this year, Carbon 3D’s DENTCA Denture Base II and DENTCA Denture Teeth, 3D printable resins for the dental industry, received Class II approval from the FDA.
Since the Cures Act’s clarification on software as a medical device, Materialise Mimics Innovation Suite used for 3D printed anatomical parts for diagnostic purposes, became the first medical software to receive Class II certification from the FDA.
For more news on 3D bioprinting and advances in medicine subscribe to our 3D printing newsletter. Also join us on social media: Twitter and Facebook.
Looking for a new career? Visit our 3D printing job site.
Featured image shows the surface structure of an FDA approved 3D printed cellular titanium implant. Image via EIT.

