A team of engineers from the Delft University of Technology has used extrusion-based 3D printing to fabricate temporary bone implants made of porous iron.
Much like magnesium or zinc, porous iron is biodegradable, meaning it has great potential as a temporary bone substitute that serves to degrade as new bone regrows in its place. By reabsorbing into the body, temporary implants alleviate the risk of long-term inflammation, which is typically associated with permanent bone implants made of metals such as titanium.
Amir A. Zadpoor, the lead author of the study, states, “In comparison with other biodegradable metals or polymers for bone implants, iron has a high mechanical strength, which allows for the design and fabrication of porous structures for the treatment of critical bone defects.”
An unlikely technology choice
At first glance, it seems like it would make perfect sense to 3D print bioresorbable bone implants using iron. The metal is used by the body to transport oxygen, it accelerates certain enzyme reactions, plays a major role in immune response, and is a key ingredient of bone regeneration.
The issue arises when looking at the most common form of iron, which is bulk (dense) iron. Unlike porous iron, bulk iron has a very low biodegradation rate, owing to its relatively small surface area. Unfortunately, previous attempts to 3D print porous bone scaffolds with technologies such as powder bed fusion have been met with limitations, mainly related to accessibility, costs, or porosity control. While extrusion-based 3D printing can jump some of these hurdles, it is often synonymous with lower-quality parts that are not suitable for end-use medical products.
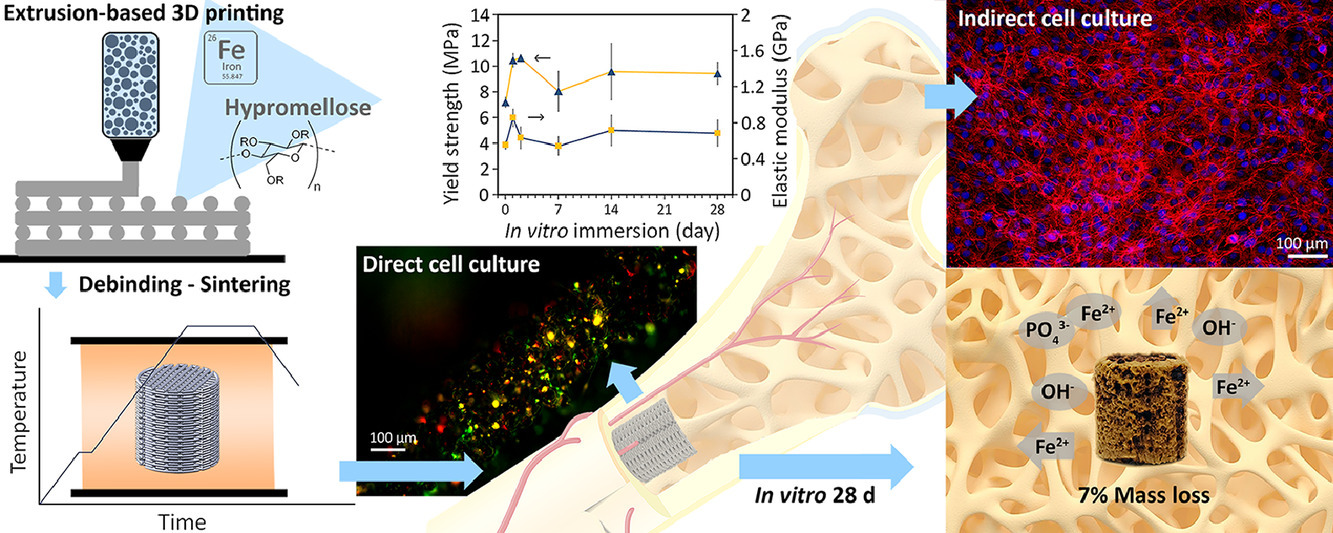
As such, the Delft team opted to try out their own purpose-built extrusion-based setup. Zadpoor adds, “We wanted to verify the feasibility of applying extrusion-based 3D printing to fabricate porous iron and explore the potential of resolving the fundamental issue of bulk iron, which has a very low biodegradation rate while maintaining other important properties such as structural integrity and mechanical properties during the bone healing period.”
7% every 28 days
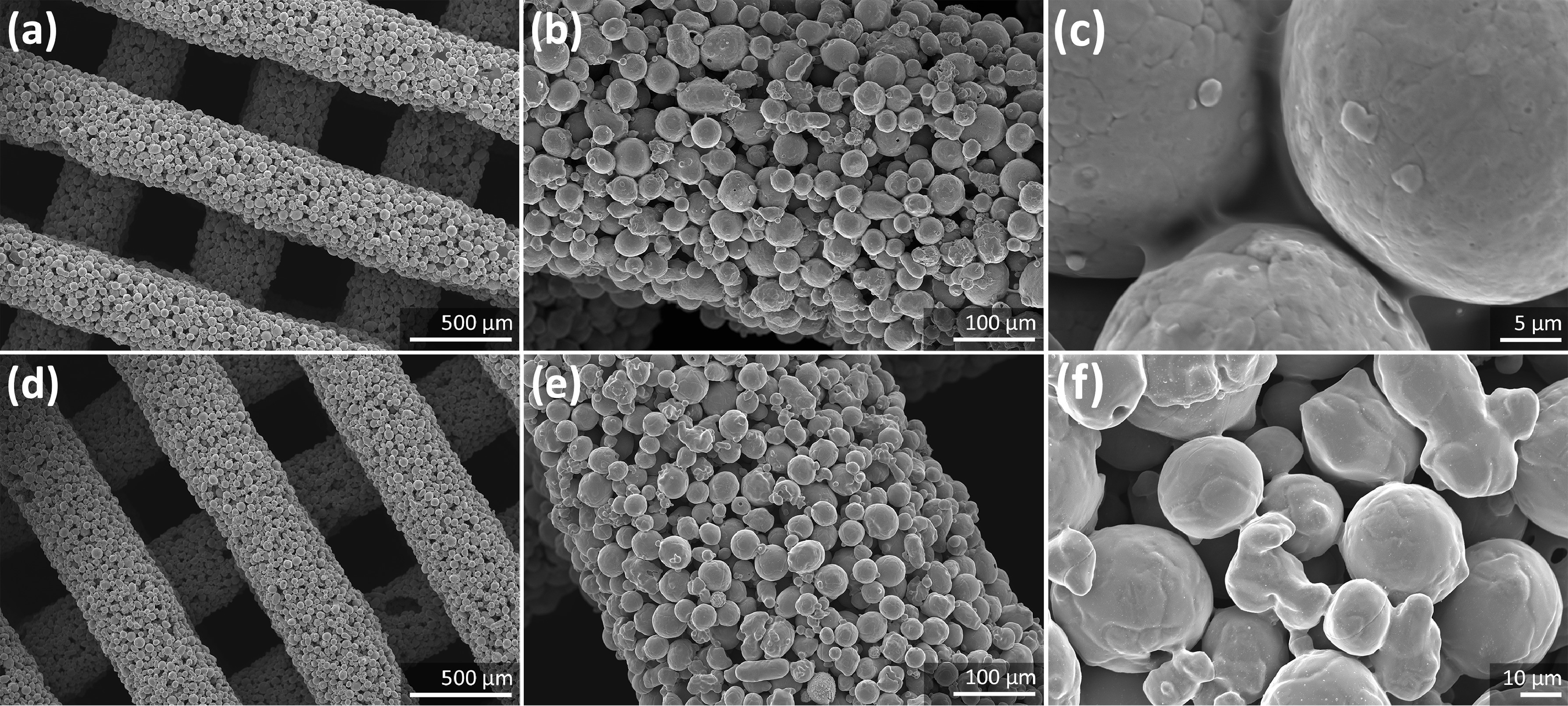
The team’s approach involved infusing a polymer-solvent with iron particulates, creating a composite ink. Once the scaffold structures were printed, they were heated to burn away the polymer, leaving the iron behind. The remaining iron structures were then heat-treated further to sinter them into porous solids.
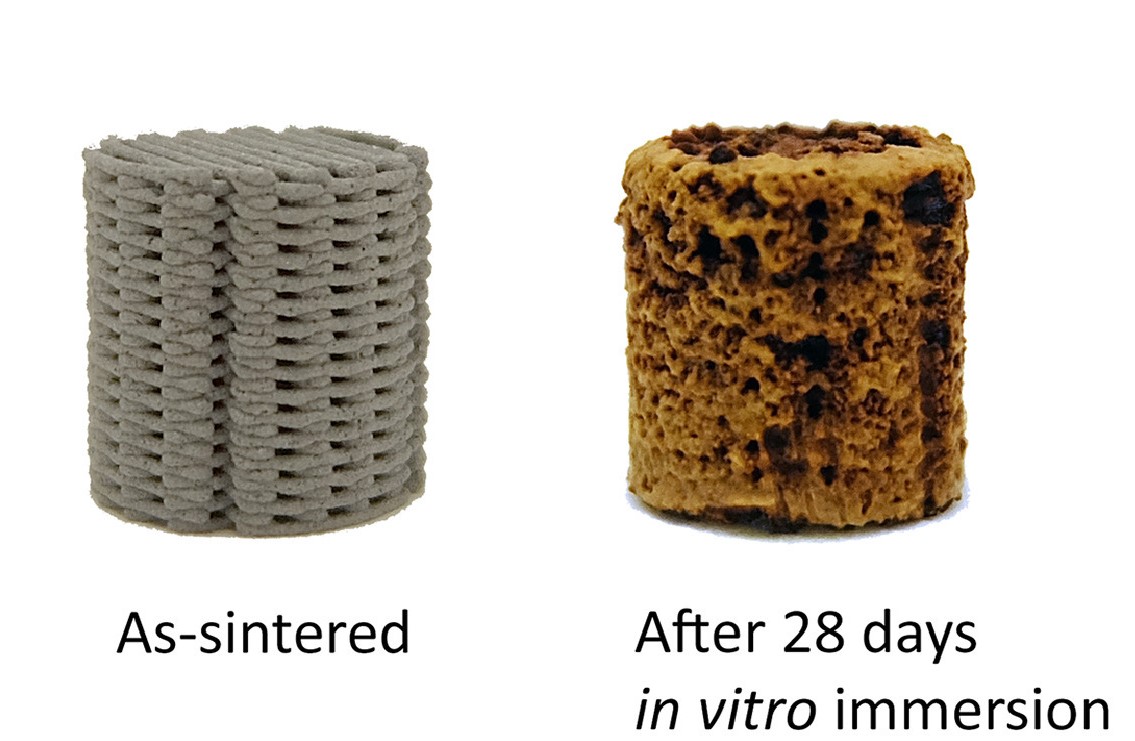
Zadpoor and his colleagues immersed their printed porous scaffolds in specially formulated simulated body fluids and determined a very high biodegradation rate – the iron lost 7% of its mass every 28 days. The team also found that corrosion had occurred around and even inside the implants, but their mechanical properties remained with the range of human bone.
“We have confirmed that extrusion-based 3D printing can deliver porous iron scaffolds with enhanced biodegradability and bone-mimicking mechanical properties for potential application as bone substitutes,” stated Zadpoor.
The next stages of the research involved testing the potential of extrusion-based 3D printing for other, more advanced implant-related functionalities. This involves the infusion of nanobioceramics to promote bone growth, and even antibacterial agents to alleviate the risk of post-surgery infections.
While titanium is largely regarded as the go-to bone-implant metal, additive manufacturing researchers are constantly proving the suitability of other materials for the application. Late last year, scientists from the Skolkovo Institute of Science and Technology developed a novel method of 3D printing personalized bone implants made of ceramic. Much like the Delft implants, the Skolkovo implants featured large pores, enabling them to better fuse with organic tissue.
Elsewhere, researchers have previously developed nanoclay-based 3D bioprinted scaffolds that were used to aid skeletal regeneration. The bone-substituting scaffolds were infused with human bone marrow stromal cells and human umbilical vein endothelial cells, further encouraging bone growth.
Subscribe to the 3D Printing Industry newsletter for the latest news in additive manufacturing. You can also stay connected by following us on Twitter and liking us on Facebook.
Looking for a career in additive manufacturing? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured image shows an in vitro biological evaluation of the iron scaffolds towards preosteoblasts MC3T3-E1. Image via TU Delft.


