The Québec Industrial Research Centre (CRIQ), has installed a GE Additive Arcam Electron Beam Melting (EBM) Q10 Plus machine at its facility to accelerate the development of patient-specific lower jawbone implants.
As part of its medical partnership with the University Hospital of Quebec (CHU de Quebec), CRIQ is working with CHU’s cranio-maxillofacial surgeons on applying additive technologies to transform the design to manufacture process of such implants.
“The medical device industry is one of the pioneer industries of additive manufacturing,” said Stephan Zeidler, Business Development, Manager Medical, GE Additive.
“[It] enables companies to manufacture patient-specific implants and customized devices in small batch production, but still in a cost-effective, industrial process. This way, the technology perfectly serves the trend for more individualized treatments in healthcare.”
Quebec invests in additive manufacturing in healthcare
In 2016, CRIQ entered into a medical partnership with CHU – which included five hospitals in its province – to improve medical technologies for better patient care. During this year, CRIQ also partnered with the Canadian Manufacturers and Exporters (CME) to leverage the use of additive manufacturing in Canada.
The following year, CRIQ and the CHU network launched a project to open a $2.874 million (USD) medical 3D printing center in Québec City. This facility will be integrated into all hospitals within the region to provide point-of-care 3D printing services for customized prosthesis as well as research in the field of regenerative medicine.
As a result of its ongoing work with cranio-maxillofacial surgeons, led by François Gingras, Director of Industrial Equipment at CRIQ, the institutions intend to improve the entire design and process cycle as well as validation and medical certification for patient-specific lower jawbone implants. Specifically, the teams are aiming to provide a three-week turnaround of patient-specific implants, rather than the current six weeks required when using traditional manufacturing.
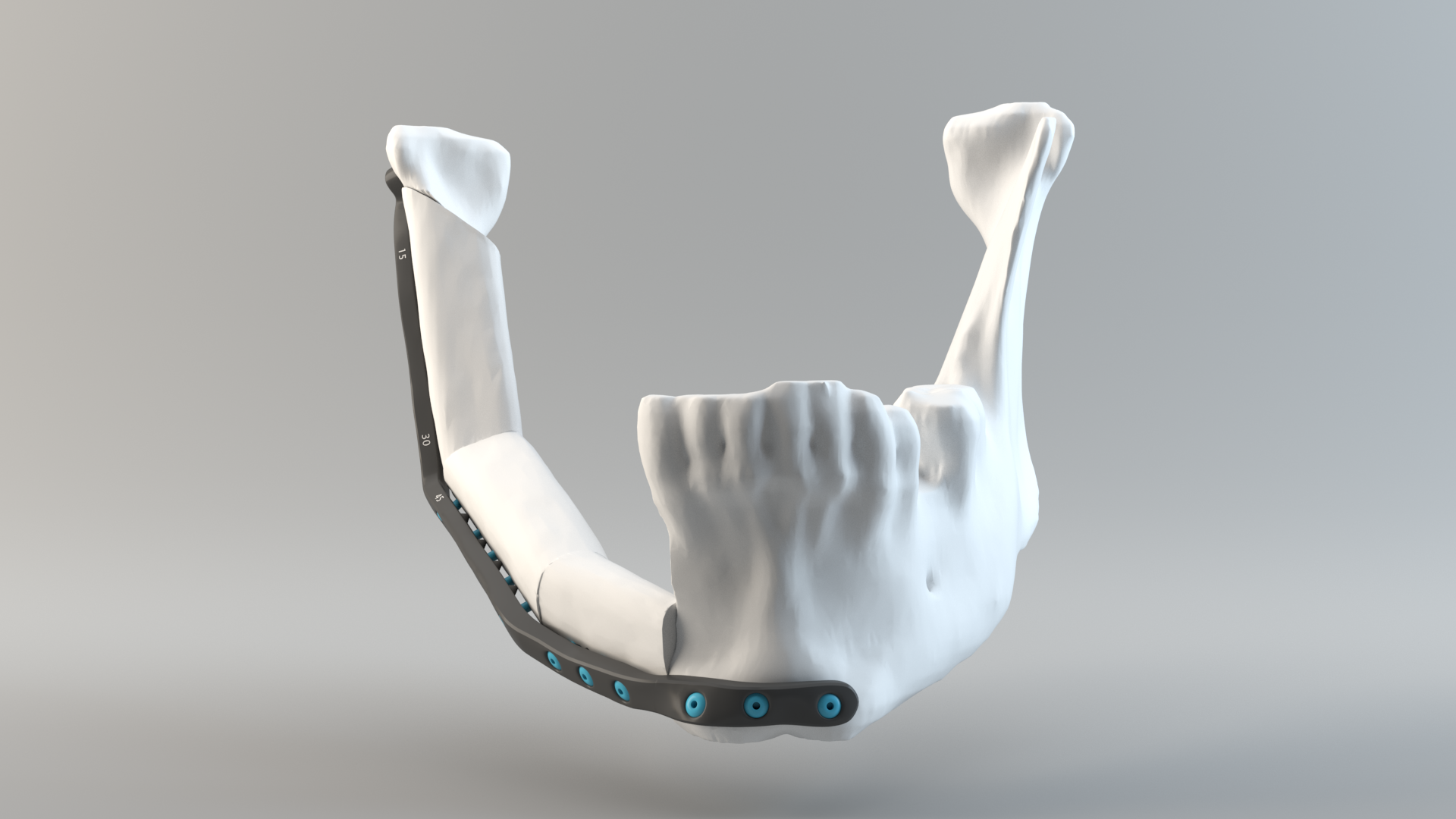
3D printed, patient-specific lower jawbone implants
CRIQ and CHU are tackling the entire supply chain which covers design and CAD of implants, fabrication of the implant, post-processing, cleaning, and sterilization. According to CRIQ, this strategy will define and influence pre-processing, design, testing, and fabrication systems and ensure consistency and repeatability.
Gingras explains that this approach challenges the perception that patient-specific implants are too expensive and not a good business case for additive manufacturing. “CRIQ sees the business case for patient-specific implants at a system level as they cannot be measured on an implant by implant basis.”
“The business case isn’t in a part-to-part comparison; it needs to be justified through system-wide impact. If a patient-specific implant can accelerate patient recovery, reduce risk, and lower overall healthcare costs for the Quebec Government, then we have a business case,” added Gingras.
CRIQ is expected to enter full production of its 3D printed implants by January 2020 upon completion of the process of medical certification of additively manufactured mandibles by Health Canada.

Stay updated with the latest in additive manufacturing by subscribing to the 3D Printing Industry newsletter. Also, follow us on Twitter, and like us on Facebook.
Looking for a change of pace or seeking new talent? Search and post 3D Printing Jobs for opportunities and new talent across engineering, marketing, sales and more.
Featured image shows a 3D model of a lower jaw implant. Photo via Quentin Schneider/ 3D modeling lab, CHU de Quebec.

