Research at the Center for Engineering Photonics, Cranfield University in Bedfordshire UK has developed a method for FFF 3D printing microfluidic devices. Using a commercial 3D printer, the method could drastically improve the accessibility of microfluidic research, typically created by 3D bioprinting or vat-polymerization techniques.
A paper covering the results of Cranfield’s method is published in the Journal of Micromechanics and Microengineering, and co-authored by Alexander Tothill, Matthew Partridge, Stephen W James, and Ralph Tatam. It is openly accessible to anyone viewing the link, and a model of the 3D printed device is downloadable from Thingiverse – upholding the value that anyone should be able to give it a try.
We asked Dr Matthew Partridge, research fellow at Cranfield University, to give us an insight into the study, and its possibilities for the development of microfluidic research.
An introduction to microfluidics
The study of microfluidics is intriguing to scientific research and technology because of its submillimeter precision, and ability to automatically control fluids within a space typically the size of microscope slide.
In microfluidic devices, researchers are able to accurately monitor the effects of liquids in contact with each other. This is useful for diagnosing the behavior of cells, and even manipulating the flow to help administer drugs within the body.
Existing methods of 3D printing microfluidic devices
Because of the precision capable with a nozzle and computer-aided design (CAD), 3D printing is often explored as a method of making microfluidic chips.
When creating a gelatinous microfluidic device with living cells, 3D bioprinters are used, as in the kidney lab-on-a-chip research at Harvard University. For rigid microfluidic chips, vat-polymerization techniques like SLA, DLP and CDLP are also generally favored for their high-resolutions, typically when making a mold for such a device as in the microfluidic LEGO bricks.
FFF is the most readily available and cost-effective method of 3D printing. Additionally, as it is typically made from corn starch, PLA filament is biocompatible. And so, to open up access to microfluidics, and increase its potential impact, it makes sense that effort should be put into developing a way to make the devices in this way.
Making microfluidics on a 3D printer
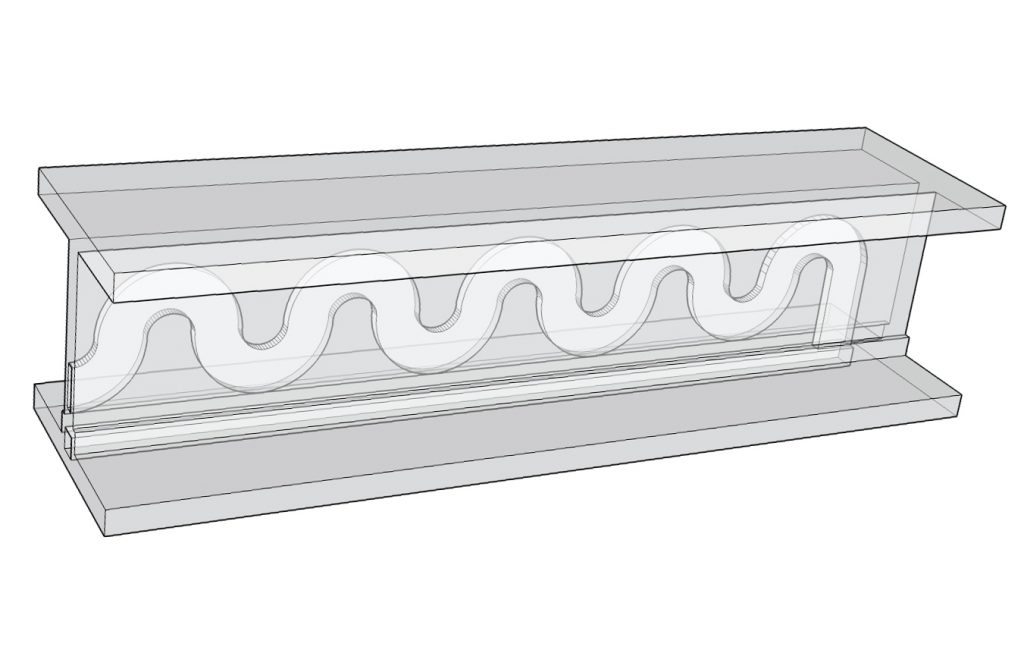
The research put forward by Ralph Tatam et al. in Fabrication and optimisation of a fused filament 3D-printed, demonstrates the use of two girder-shaped cuvette vessels 3D printed in clear PLA filament.
The first cuvette is 3D printed with straight channels to test the flow of liquid through the material. And the second cuvette is designed with a snaking channel at 500 μm (micrometers) in diameter – the equivalent span of 10 human hairs.
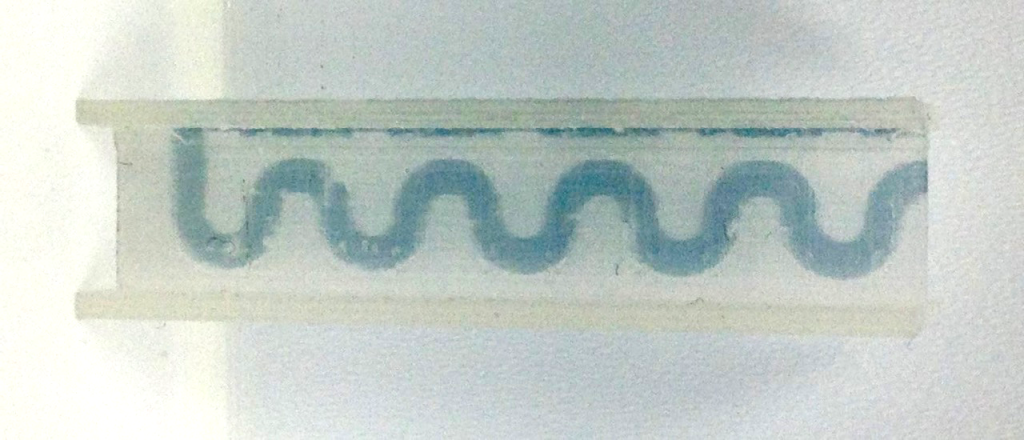
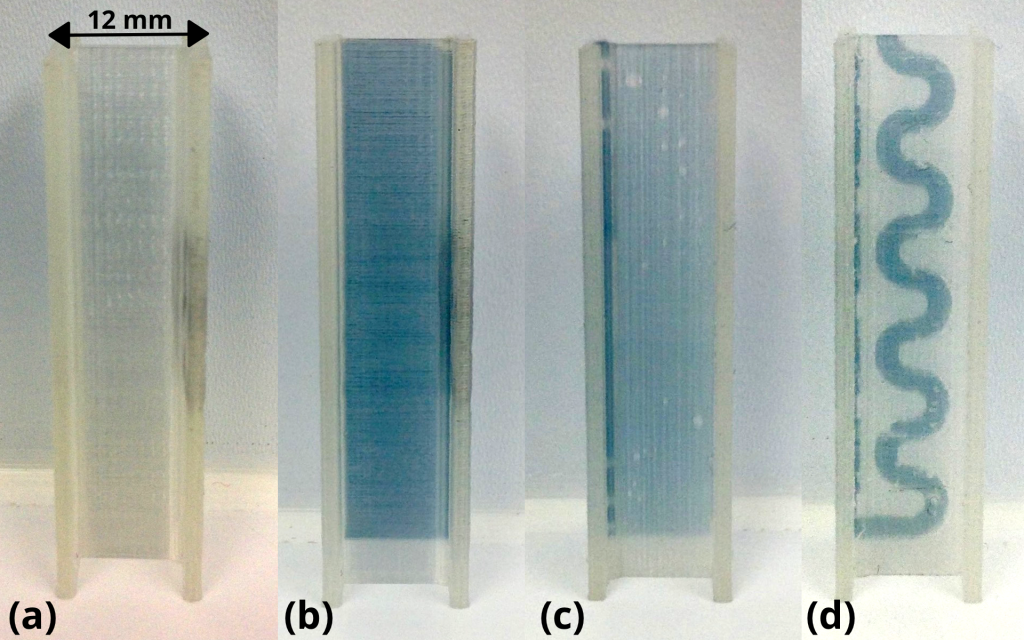
The cuvettes were tested with a dye solution, showing that the fluid is capable of effective movement through all channels of the device. The dye was injected into the rectangular opening of the cuvette as seen in the image below.
In the snaking device, the liquid is then carried through all the bends in a motion similar to the way that u-bends work to flush water out of a sink.
With the idea of 3D printing the S-cuvette at home, we asked Dr. Partridge what he would recommend as a household experiment, he explains:
If you put a little drop of food dye into the top of the S curve (be careful not to block the straight channel).
Then to help the flow (the plastic is hydrophobic so needs a little help) put it under a running tap holding it at about 45 degrees with the straight channel hole at the top and outside the water (if possible) and the S hole under the water.
It should push it nicely through making a dye front (quick snap attached) showing the snaking path.
Adding:
I’ve not got any to hand but I think it also might work well with coloured milk. The proteins might overcome some of the hydrophobicity.
Hence where our ‘crazy straw’ idea comes from.

Seeing things clearly
To improve the optical quality of the cuvettes, researchers tried acetone treatment, finding it improved the clarity of the material by 20%. However, as Dr. Partidge explains:
What we are showing in the paper is that FFF 3D printing of microfluidics can be made to work without any post processing. The images in the paper are all of microfluidics run straight off the 3D printer, no acetone, no polishing, no heat treatment.
Dr. Patridge adds that getting acetone vapour into the channel to effectively clear them is a tricky task. The PhD student Alex Tothill who conducted the majority of the research featured in the paper has been working exclusively on the process since 2015. Tothill’s research continues to look at other methods of improving clarity in a way that is compatible with microfludics.
For more first hand insight into research using 3D printing, you can get updates from 3D Printing Industry in our newsletter, or via Facebook and Twitter.
Featured image shows a stack of plastic ‘crazy straws’ suggestive of the S-shaped flow of Cranfield’s microfluidic device. Photo by Ashely Moore of lifewithmoorebabies on Blogspot


