Researchers from the University of Illinois at Chicago (UIC) have developed a scaffold-free 3D bioprinting process.
Commonly, biodegradable scaffolds are used to maintain the shape of 3D bioprinted tissue used in regenerative medicine research. However, according to the study published in Materials Horizons, degradation byproducts within the scaffolds can be toxic as well as interfere with the development of cell-to-cell connections of functional tissues.
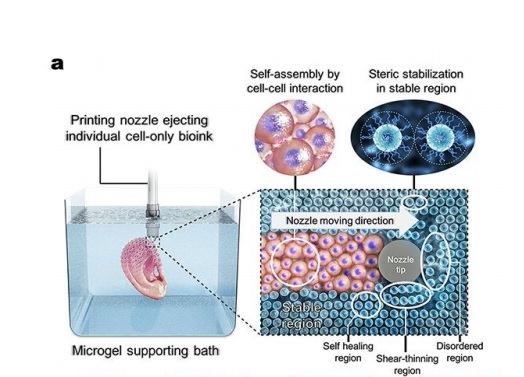
Ay UIC a microgel supporting bath has been created to allow free movement of a printing nozzle for high-resolution cell extrusion. Eben Alsberg, Richard and Loan Hill Professor of Bioengineering and Orthopaedics, UIC, explained:
“Our cell only printing platform allows for the 3D printing of cells without classical scaffold support using a temporary hydrogel bead bath in which printing takes place.”
“For the first time, cell-only constructs can be printed in intricate forms that are made up of different cell types without a hydrogel carrier or traditional scaffold that can then be stabilized for a period of a day to weeks.”
A hydrogel bead bath
In the UIC research, micron-scale hydrogel beads making up a bath allow a 3D bioprinter nozzle to move and deposit cells in place while preserving their shape. These cells are exposed to UV light, which cross-links or “freezes” the beads together, ultimately enabling them to mature and grow within a stable structure.
“The hydrogel bead bath has unique properties which allow for both printing of the cell-only bioink in complex architectures and subsequent temporary stabilization,” added Professor Alsberg. “Using chemistry we can then regulate when the beads go away.”
With the hydrogel bead bath, the UIC team used stem cells to 3D print cartilage in the shape of an ear and a rodent-sized femur, forming stable, cell to cell connections through specialized proteins.
Through the use of a sacrificial support gel, instead of scaffolds the method has some similarities with FluidForm’s FRESH 3D bioprinting methodology, and microfludic chip fabrication conducted by the Lewis Lab at Harvard University.
3D bioprinting large functional tissues
Although 3D bioprinting with scaffolds provides support for the underlying architecture of an organ or tissue by seeding cells, scaffold decomposition can be difficult to time in tandem with the maturation of said organ or tissue.
Following the success of the 3D bioprinted ear cartilage and femur, Professor Alsberg said, “We’ve demonstrated that individual cells and cell aggregates can be organized and assembled using this platform strategy to form larger functional tissues.” This process is expected to be valuable for tissue engineering, drug screening and as models to study developmental biology.
“Individual cell-only bioink and photocurable supporting medium for 3D printing and generation of engineered tissues with complex geometries“ is co-authored by Oju Jeon, Yu Bin Lee, Sang Jin Lee, Derrick Wells, Hyeon Jeong and Eben Alsberg.
Want the latest additive manufacturing news? Subscribe to the 3D Printing Industry newsletter and follow us on Twitter and Facebook.
Looking for a career in additive manufacturing? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured clip shows the bioprinting process of the letter “C” using a stem cell only ‘bioink’ into an alginate microbead supporting medium. Clip via Oju Jeon and Eben Alsberg/UIC.

