Research from the University of Greifswald, Germany, uses quinine, a substance found in tonic water also used to treat malaria, to study 3D printer filaments for their ability to gradually release drugs into the body.
The idea is to use the materials to make smart implants, for example in hip replacements or spinal surgery, that support bone regrowth while releasing medicine at the point of inflammation and pain.
Quinine is the tonic
Researchers at the University of Greifswald select four polymers for the study based on their bio compatible and degradable properties:
- Eudragit®RS – popular for making oral dosage pills
- Polycaprolactone (PCL) – biodegradable polyester
- Poly (L-lactide) (PLLA) – a polymer related to PLA
- Ethyl cellulose (EC) – a thin film material related to glucose
Through a process of solvent casting, the polymers are dissolved into a solution. Quinine is then added to the solution as the active drug. As it is luminescent under ultraviolet light, the quinine can also be measured easily as it is released.
Once the quinine is mixed in, the solvent part of the solution is vaporised leaving a polymer/quinine film that is cut into a filament. This filament is then fed through a self-made extruder into cylindrical sample implants.
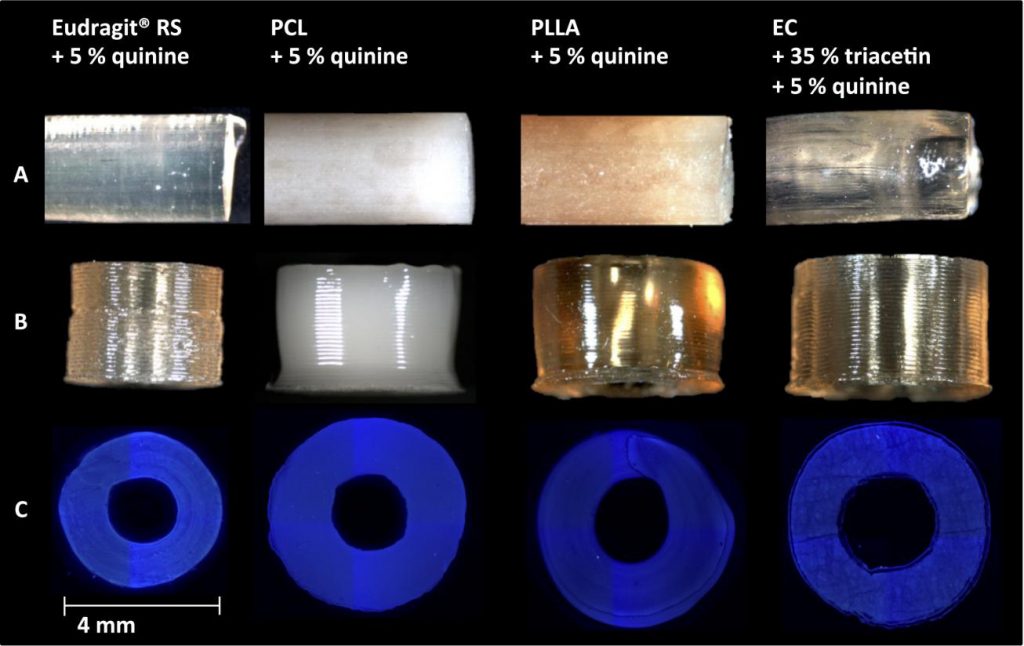
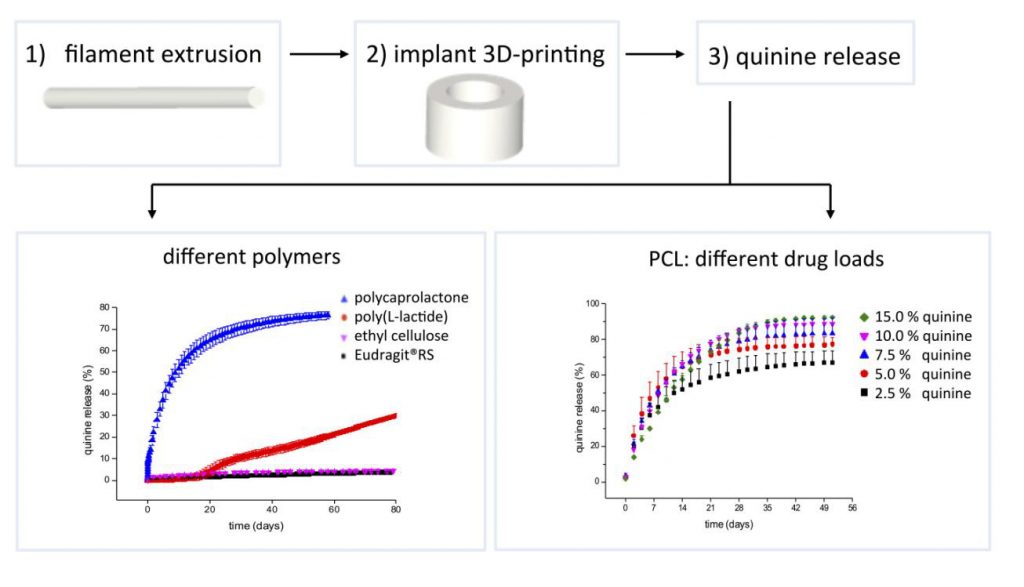
Controlling the release into the body
Over a test period of 100 days in the lab, Eudragit® RS and thin-film EC showed the slowest rate of release at only 5% of the contained quinine.
In only 51 days, the biodegradable polyester (PCL) showed the most rapid rate of drug release at 76% of quinine. This lead researchers to further experiment with PCL.
Results showed that, “….increasing the drug load also increased the overall percentage of drug released to the medium since nearly the same absolute amount of quinine remained trapped in PCL at the end of drug release studies.”
3D printed pharmaceuticals
In developments for specific and personalized treatments for patients, 3D printing is poised to transform the pharmaceutical industry. Tailor-made by nature, and already made using 3D printing by companies such as Stryker, implants containing drugs are moving ahead of the 3D printed pill.
This particular study has proved valuable for continued research, and is conducted by Wiebke Kempin, Christian Franz, Lynn-Christine Koster, Felix Schneider, Malte Bogdahn, Werner Weitschies and Anne Seidlitz from the Center of Drug Absorption and Transport at the University of Greifswald.
The paper titled Assessment of different polymers and drug loads for fused deposition modeling of drug loaded implants was accepted into the European Journal of Pharmaceutics and Biopharmaceutics on 17 February 2017 and can be read in full here.
To stay up to date with 3D printing research you can subscribe to the 3D Printing Industry newsletter here and follow our active social media channels.
Featured image shows a wave of suspended, glowing bottles containing tonic water – “Tectonic” artwork by Sean Virili, Justin Hartany, Matt Fung & Jamie Bastoli. Photo by Flickr user 78821753@N03


