For every star performing biotech, life sciences or innovative 3D printing company there are another 9 where investors would have been better off keeping their cash under a mattress.
As Organovo report their first full year operating on a commercial basis we look for clues as to which category they might fit into. With more than 25 patents secured and another 80 pending, do the current share price and today’s published financial accounts tell the full story?
Organovo increased total revenue from $570 thousand in 2015 to $1.5 million for 2016. However, losses also increased from $30.8 million to $38.6 million. Although yet to turn a profit, Organovo were always going to generate a sizeable amount of text in the 3D Printing media and beyond. The promise of combining biophysics, developmental biology and of course 3D printing to advance healthcare and life sciences is an attractive proposition.
Globally, annual revenue in the pharmaceutical industry exceeds $1 trillion. If the industry were a country it would rank as the 16th largest by GDP, only marginally behind Mexico. So when Organovo CEO Keith Murphy says, ”we are serving critical unmet needs in markets with very attractive profiles” this may be something of an understatement.

On Thursday’s call with analysts and investors Murphy reiterated a point he has made a number of times before. Today’s total revenue is yet to reach the potential annual income of $200m* that is possible within the near-term.
As the founder of Organovo, Dr. Gabor Forgacs has received accolades and numerous conference speaking invitations. Earlier this week, at one such conference, Forgacs explained to the audience at the 8th edition of the From Solid State to Biophysics conference in Dubrovnik, Croatia one of these unmet needs in an attractive market.
“$65 billion dollars. This is the amount spent by the global pharmaceutical industry in the U.S. during 2011 on research and development”, said Forgacs. This resulted in the approval of 21 drugs by the FDA, which equates to about $3 billion per successful drug.
The low success rate can be explained in part by the transition between preclinical animal trials (which themselves are preceded by substantial work) and human clinical trials. This transition sees between 20-50% of drug candidates fail. The preclinical work typically equates to a sum that can reach $500 million. Forgacs says the simple explanation for these expensive failures are the differences between animals and humans.
Organovo believe they have a solution to this expensive problem: extrusion based bioprinting.
Everything Flows
Describing the process of extrusion based bioprinting Forgacs says, “You have a bioprinter and bioparticles, either spherical multicellular units (aggregates) or cylindrical units. The print nozzle lays down these particles together with a supporting material that is later removed. Once the particles are laid down, they fuse. The composition of the bio-particles depends on what you want to do.”
While other approaches to regenerative medicine such as inkjet bioprinting and using chemically infused scaffolds exist, Forgacs believes the extrusion method is the most powerful.
In contrast to 3D printing in the inanimate world Forgacs explains, “bioprinting does not result in an (immediately) useful product, a viable biological structure without post-printing maturation.” Maturation involves several processes, at this stage biology takes over and the living cells fuse together. Forgacs gives an example of vascular structures such as blood vessels, “The bioprinted structure is put into a profusion bioreactor where near physiological conditions are created. Under these conditions the structure starts maturing.”
Maturation is a, “highly non-trivial process”. To form a blood vessel, for example, three specific cell types are required. He continues, “So something miraculous happens during this post printing maturation process. Not only do the cells fuse but the cells sort out. i.e. they find their physiological location and interface between the flow and the rest of the structure.” This is nature’s amazing power to self-organize. Forgacs explains, “this is because there is (blood) flow. The cells have stress sensitive genes that sense the flow and as a consequence they are forced genetically to go where they are supposed to go. That this happens in-vitro, that is outside the body, is mindboggling”
This tissue fusion and cell sorting-out are the two most important phenomena at work here.
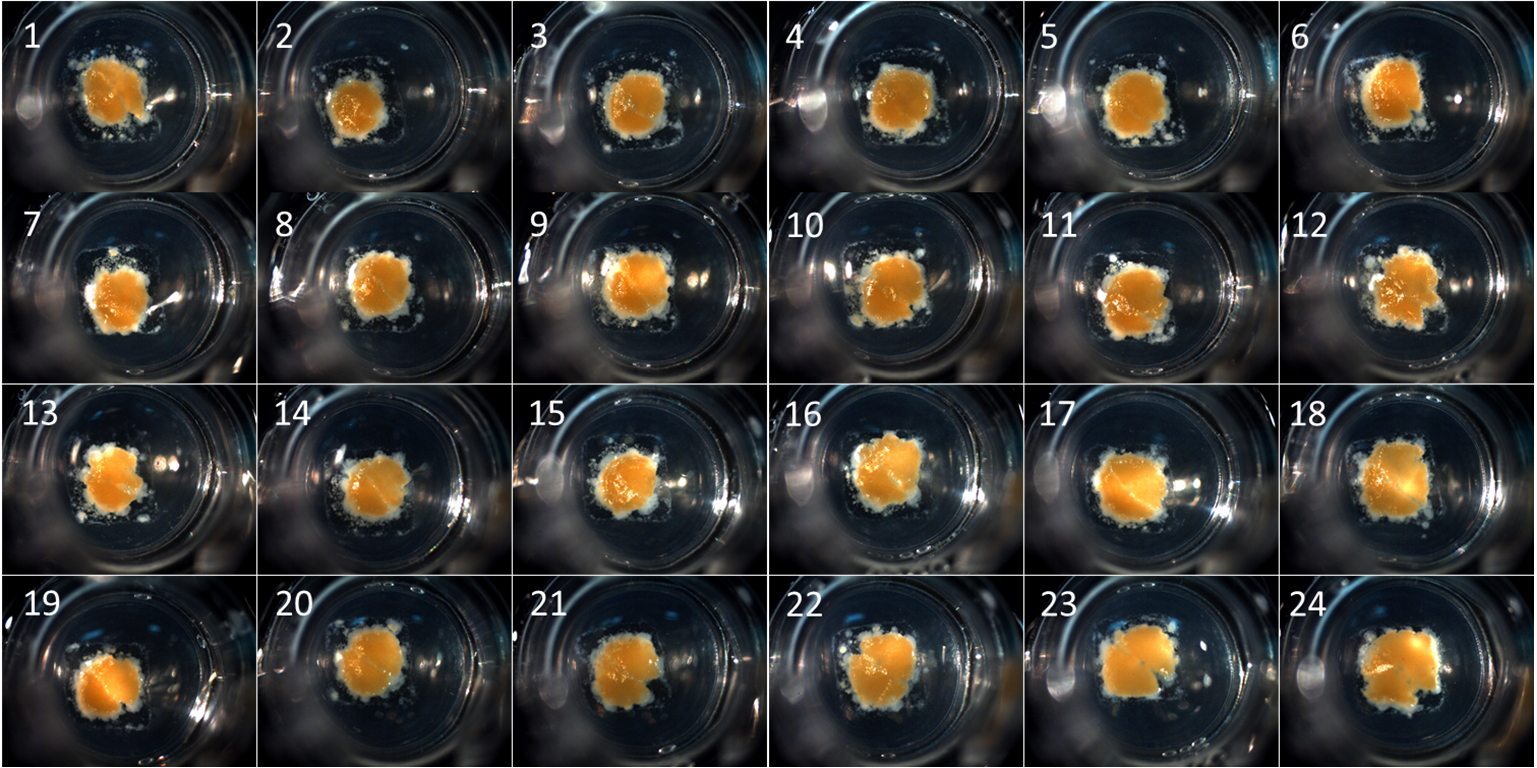
Another key component of the underlying science of bioprinting is based on tissue liquidity, pioneered by the developmental biologist Malcolm Steinberg at Princeton University. Steinberg realized embryonic tissues have a lot in common with liquids. Forgacs explains, “irregular tissue fragments spontaneously round up into spherical aggregates, liquid drops do the same thing. Embryonic tissues preferentiality envelop each other (or sort out) like liquids which have surface tension, water has a higher surface tension than oil which is why they remain separate with the oil surrounding the water. This can be reproduced in embryonic tissues.”
It is must be pointed out that this is an analogy, not an identical process, but it allows scientists to make predictions.
From the lab to real world
Organovo want to use their bioprinted structures, which are engineered to be structurally and functionally equivalent to humans, as an interface between preclinical animal trials and human clinical trials. This will provide an earlier indication of the success of new drugs. Eventually, Forgacs envisions bioprinted structures may remove the need for controversial animal trials entirely. As CEO Keith Murphy puts it, “our competitive advantage is that we make 100% cellular functional human tissues.”
Organovo’s first commercial product, the exVive3D Human Liver Tissue, launched in November 2014 and drove the bulk of the $570,000 revenue for the financial reporting period ending in March 2015.
Organovo previously presented the results of tests, which validate their claims. Murphy gave details of one test where the exVive 3D Human Liver Tissue picked up a previously missed problem. The CEO said Organovo’s product detected the “toxicity of another drug missed during preclinical studies, troglitazone, which was marketed as Rezulin and caused acute liver failure in 100s of patients. The drug had a clear toxic response in the exVive3D Liver Tissue at seven days.”
Murphy explains a core advantage of Organovo’s product is addressing gaps of other testing methods. He says that in the liver space, predominantly animal models and 2D cell cultures are the gold standards. Some use other technologies from 2007/2008, but these are only used by 1-2%. As a first mover Organovo have a clear competitive advantage.
To speak at a conference entitled From Solid State to Biophysics is very appropriate for a theoretical physicist turned tissue engineer. Indeed, Forgacs wears a number of different hats. He began his research into bioprinting at the University of Missouri-Columbia, this was spun out into Organovo and then Modern Meadow.
In 2005 Forgacs began to gear up his lab at Missouri for bioprinting research, and a number of his academic research team followed him in 2007 when he left to found Organovo. The company now employs 128 people at its laboratory in San Diego and Forgacs makes frequent visits to Brooklyn, New York where Modern Meadow, founded 2013, is based. In the same year Organovo listed on the NYSE MKT stock exchange.
Shortly after listing Organovo’s share price reached a $12.50 high point in November 2013. Currently the price is hovering below the $3 mark and the company has a market cap of $260 million. A flurry of trading activity (a possible short-squeeze) in early April raised the price to its current level, previously the stock had reached a 12 month low of $1.60.
Analyst sentiment regarding Organovo will be bolstered by these latest results. Although, even during past bearish phases analyst opinion has prevailed that the stock remains a hold or buy recommendation.
Addressing the vagaries of equity markets retired CFO Barry Michaels had previously said, “This is a business that has to grow year-over-year not necessarily quarter-over-quarter and we need to make and demonstrate steady progress towards that goal of getting it into the tens and millions.” Michaels completed his last day at the company in April 2016, the search for a replacement CFO is ongoing.
Analysts have occasionally questioned this pace and pointed to the company’s periodic returns to equity markets for additional funding. A necessity that has allowed operations to continue while losing $10 million a quarter, but rarely a signal received in a positive light by the market.
During 2016 Organovo raised $43.1m (2015: $23.10m) from stock issuance. CEO Murphy was keen to stress the company’s willingness to use non-diluting forms of finance such as grants and partnerships. The partnership with L’Oreal to develop a synthetic skin product is one example of this.
With reported cash and equivalent balances at $62 million for the fiscal year ended March 2016 ($50m 2015) based on historic performance the company will be able to operate for 2 years before requiring an additional cash infusion.
“You can live forever”
Organovo can already print elementary organ structures like vessels and make small pieces of tissue. This year the company will release a kidney tissue and then another for heart tissue in the future.

The next stage is to put together the liver, kidney and heart tissues connected by blood vessels. This will allow testing in an environment that even closer replicates that inside the human body. These models of organisms with 5 or 6 tissues connected together that function in unison will be a compelling product for pharmaceutical companies.
Moving even further forward Forgacs believes that the small replica livers can be implanted into those awaiting organ transplant. This will sustain patients until a suitable donor can be found. He refuses to speculate on the issue of whether full organs can be bioprinted.
He warns, “we will never be able to create the exact same copy of very complex human organs like the heart” then adds, “but who says we need to?”
The heart is a pump and, “if we can make a pump from your own cells using bioprinting it can have the same function, it will be immunologically compatible with your body, and if you want, you can live forever.” This will be possible, he says, within 50 years.
Beyond regenerative medicine Forgacs envisions bioprinting applications for meat and leather. If the company can make medical grade tissues what if it could also make meat and leather with lower inputs of land, water, energy, chemicals, and no harm to health and animals. Modern Meadow was established to pursue this goal.
The Modern Meadow process works by taking cells via bioposy, the cells are grown in a lab and then the useful cells isolated. As 95% of leather is composed of collagen, these are the cells isolated for that particular application. A structure is then created using bioprinting and the result can be tanned and used as leather. Making leather in this manner allows pieces to be made for purpose and therefore decreases the waste that occurs when using the product in its natural and often irregular form.
Bioprinting for meat and leather uses primarily planar structures, which are easy to form with 3D printing. The leather can also be made substantially finer which makes it more suitable for use in fashion garments in addition to more utilitarian items of clothing such as protective jackets. In the food area, Modern Meadow have created a meat chip that is 60% animal protein, no other product has this level of animal protein.
Forecasting the Future
Expectations regarding a company’s future earnings are a key driver of its current share price. Uncertainty, or deviation from announced earning forecasts, can contribute to a price drop almost as much as a failed product or contested IP claim. There is an expectation for company executives to provide frequent market updates, primarily via answering analyst questions in detail.
Organovo break with this expectation, partly through necessity (pharmaceutical research is highly guarded, for obvious reasons) and partly through choice. Paul Gallant, general manager, joined today’s call to provide additional insight into Organovo’s sales process and customer relationship management.
Gallant confirmed that Organovo have increased from 4 to 5 the number of customers within the top 25 pharmaceutical companies. He also stated that returning customer business is strong. His core message was, “The power and versatility of our technology platform, multiple tissue types in multiple markets” and that 2017 will be a year of strong revenue growth.
Murphy explained that quarterly revenue had a tendency to be “choppy” due factors beyond Organovo’s control. As the company performs work under long term contracts these can often span quarterly reporting dates. Furthermore, the results of testing by Organovo customers may lead to changes in their internal R&D calendars and scheduling of work. Therefore, a steady increase in revenue should not be expected and its absence is no cause for alarm.
When questioned further on a breakdown of customer numbers by investment analyst Brandon Couillard of Jefferies, Murphy made the point that revenue guidance remained the best indicator and the company preferred to use GAAP metric. Murphy added that they will try to shrink total engagement time from initiating contact with customer to booking revenue. As some customers are on their 3rd orders this grants the ability to turn around the next contract faster as the customer relationship is already in place.
Biotech stocks, especially smaller capped companies, have always offered investors an interesting proposition.
A common pattern is for early excitement and a soaring stock price supported by expectations rather than financial results. Later, shares may languish as the company struggles to commercialize its revolutionary IP. Or, for the fortunate few, a windfall awaits as the company is either acquired at a healthy mark-up or flourishes in its own right.
Organovo is creating a new market and educating potential customers is a lengthy process. On average 8-11 months can elapse between initial contact with a customer and the receipt of income. But Organovo are gaining traction in the market and customers are giving repeat business. Although reasonably well known in the field of toxicology Murphy pointed out that the company must also make efforts to ensure potential customers from a wider group are also aware. He states that at a recent presentation with Bristol Myers Squibb, “more people coming to find us, our presentation was standing room only.”
Organovo are expecting a 200% growth in liver tissue business during 2017.
The new kidney product will be priced a premium and revenue recognition is forecast for the middle of the new fiscal reporting period. The company has already commenced an early access program with a dozen customers to prime the market and receive feedback. In the absence of liquidity issues a slow but steady approach appears to be a reasonable strategy.
In the long-term the tissue replacement product that Organovo hope to receive FDA approval for within 3-5 years has the potential to revolutionize patient treatment and accompanied by billion dollar market opportunities.
*$200m is based on revenue from two streams discussed by Murphy in Organovo’s Q3 2016 earnings call here.


